Introduction | What is Caffeine: Molecule Structure | Stimulating Science: The Properties of Caffeine | Discovery: The Magical Bean | Coffee Creates a Social Lifestyle in Europe |Colonization and Coffee|Coffee’s Impact on the Nation of Brazil|Coffee Industry Today| Conclusions
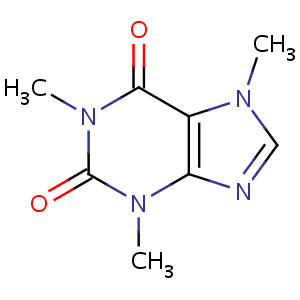
Caffeine is a naturally occurring molecule that can be found in over sixty different plants. The caffeine acts as a natural pesticide, and protects the plants by paralyzing or killing insects that attempt to feed on the plant. The most widely used caffeine containing plants are coffee, tea. The caffeine molecule is classified as an alkaloid, meaning that it is a nitrogen-based compound that is extracted from plants. The chemical formula for caffeine is C8H10N4O2, is found in coffee.
Caffeine, in its pure form, is found as odorless bitter white crystals. Pure caffeine is very soluble in hot water, which is why drinks like coffee and tea contain high amounts of caffeine. The melting point is 238° C (460° F). On average, a cup of coffee contains around 100 mg of caffeine. In contrast, the average cup of tea contains around 40 mg and the same amount can be found in a 12-ounce cola beverage.