Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
The Ozone layer is located around twelve to twenty five kilometers, or eight to fifteen miles, above the Earth’s surface. The layer protects us from the harmful UV rays by absorption. If the UV rays would get to the Earth’s surface, then life as we know it today would not exist. Also, if the UV rays can get to the surface now, it will have a severely negative impact on DNA and RNA. One billion tonnes of ozone is naturally formed and destroyed a year. Ozone is formed naturally when a UV ray breaks the bond of oxygen-to-oxygen, leaving two atoms. Then, with the help of another atom that is bonded to another oxygen molecule, the oxygen atom forms a bond to the oxygen molecule. It is naturally destroyed when a UV ray breaks one of the bonds in ozone, leaving an oxygen molecule and an oxygen atom. Each of these processes happen at the same rate, leaving a constant amount of ozone in the stratosphere.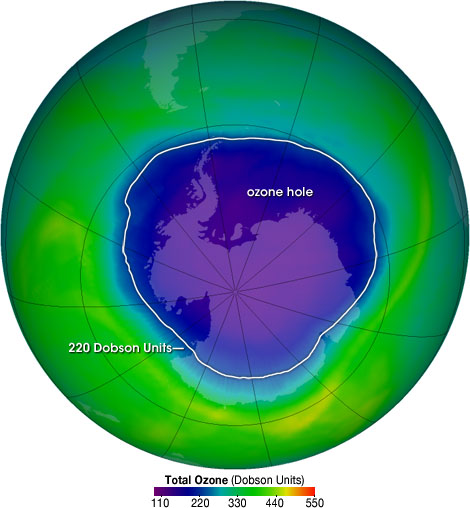 �
�
