April 20th, 2008 · Comments Off on Chemistry of Quinine ·
Intro | Chemistry | Sources | Affects History | History affects | Poor Countries | Undesired Effects | Substitutes | Conclusion
JMol Image:
Chemical Formula: C20H24N2O2

Quinine is soluble in alcohol, ether, chloroform, carbon disulfide, oils, glycerol, and acids; very slightly soluble in water. Today, it is established that quinine is made of 20 carbon atoms, 24 hydrogen atoms, 2 nitrogen atoms and 2 oxygen atoms: C20H24N2O2. The two hexagonal rings represent the quinoline structure which is common to most quinine derivatives such as chloroquine.
Categories: Quinine
Tags: Moustapha Minte —
April 19th, 2008 · Comments Off on Conclusion ·
Intro to Teflon, Serendipitous Discovery, Chemical Properties, Production, Changing History, Conclusion

Teflon has been extremely influential in many different fields. Its extremely unique properties allows it to influence everything from fabrics to the Statue of Liberty (used as an insulator and a lubricator between the copper and steel). Great advances in the US Space Program to the successful storage of extremely corrosive materials are also directly related to Teflon. Teflon was cited when President George Bush awarded DuPont the U.S. National Medal of Technology(1990) for the development of man-made polymer.
Categories: Teflon
Tags: Grant Dull —
April 19th, 2008 · 2 Comments ·
Intro to Teflon, Serendipitous Discovery, Chemical Properties, Production, Changing History, Conclusion
Teflon has changed history. Here is a list of some of the great impacts Teflon has had on our society.
1. Used for its protective attributes in the US Space Program’s space suits, heat shields, insulation and cargo liners.
2. Significantly impacted computer chip manufacturing because of its inert properties.
3. Used in nearly all tubing and piping in the semiconductor industry.
4. Teflon is used to insulate thousands of miles of communication cables due to its heat and chemical resistance.
5. In liquid form Teflon can repel water and stains from fabrics without influencing breathability, color, or feel.
6. Teflon coated fiberglass has revolutionized architecture. It is used on buildings such as Pontiac Sliver Dome, Orange Bowl, Metro Dome, and other significant structures. Teflon coated fiberglass is the most permanent of the coated architectural fabrics. It was first used architecturally in 1973 and has a life expectancy of more than 30 years. Teflon is so useful architecturally because of its anti-aging, self-cleaning, transparency, and fire retardant properties.
7. Perhaps most notably it was used to insulate valves, seals, and pipes containing Uranium Hexafluoride. This enabled the US to develop a nuclear bomb during the Manhattan Project.
Categories: Teflon
Tags: Grant Dull —
April 19th, 2008 · 1 Comment ·
Intro to Teflon, Serendipitous Discovery, Chemical Properties, Production, Changing History, Conclusion



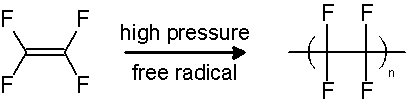

PTFE can be produced by two different processes. It can be synthesized by the emulsion polymerization of tetrafluoroethylene monomer under pressure through free radical catalyst(as seen above). Additionally, it can be created through the substitution of hydrogen atoms with fluorine on the polyethylene by using fluorine and polyethylene gas at 20 degrees Celsius.
Categories: Teflon
Tags: Grant Dull —
April 19th, 2008 · 2 Comments ·
Intro to Teflon, Serendipitous Discovery, Chemical Properties, Production, Changing History, Conclusion
Structurally there are three different types of Teflon. However, the most popular and applicable form of Teflon is PTFE; a polymer with repeating chains of CF2-CF2. The properties listed below are what makes Teflon useful in many different ways:
1. Teflon has a friction coefficient of 0.1, explaining the anti-stick properties. (Lowest friction of any known solid)
2. Teflon has a melting point of 327 degrees centigrade.
3. No water absorption.
4. Weatherability is outstanding.
5. Flame resistance LOI is 95%.
6. Resistance to chemical attack is excellent.
Categories: Teflon
Tags: Grant Dull —
April 19th, 2008 · Comments Off on Serendipitous Discovery ·
Intro to Teflon, Serendipitous Discovery, Chemical Properties, Production, Changing History, Conclusion
Like many great discoveries, PTFE was discovered serendipitously. Dr. Roy Plunkett, in a DuPont laboratory, was working on freon gas related to refrigeration when he accidentally discovered PTFE (1938). He accidentally froze a sample of tetraflouroethylene, and after checking it he discovered it had polymerized into what is now known as Teflon. It would be first sold in 1946, giving Dupont a dominating edge on competition.
Categories: Teflon
Tags: Grant Dull —
April 19th, 2008 · 3 Comments ·
Intro to Teflon, Serendipitous Discovery, Chemical Properties, Production, Changing History, Conclusion
Teflon or polytetraflouroethylene (PTFE) is a synthetic flouropolymer that has many uses. It was created and popularized under the brand Dupont, who then dubbed PTFE Teflon. It is undoubtedly most well know for the anti-stick and inert properties. Teflon is essentially inert to all chemicals and often considered the slickest material in the world. Teflon has greatly influenced areas such as: aero-space, electronics, fabrics, communications, along with many industrial processes. Teflon’s first significant use was in the Manhattan Project, where it was used to contain highly reactive uranium hexafluoride.
Categories: Teflon
Tags: Grant Dull —
April 19th, 2008 · Comments Off on GRD ·
Categories: Teflon
Tags: Grant Dull —
April 19th, 2008 · 2 Comments ·
|Introduction|Structures and Properties|Ancient Anesthetics|From Co to Pro: History Affects|
|Beyond the Dentist Chair: Novocaine Affects|Allergies|References|
A throbbing toothache has most likely ended patients in the dentist chair one time or another. Just as the chair reclines and the drill gets fired up, patients quickly request the magic injection that numbs the approaching pain! “Give me, NOVOCAINE!”
Scientifically known as procaine hydrochloride, Novocaine is the commercially accepted name of the anesthetic. The synthesis of Novocaine was monumental in the medical field, evolving anesthetic practices from their more dangerous drug roots (i.e. cocaine). The turn of the century discovery of Novocaine and it’s numbing powers has progressed the anesthesia industry and led to the discovery of similar, but more advanced drugs.
Categories: Procaine (Novocaine)
Tags: Elizabeth Gekas —
April 18th, 2008 · Comments Off on References ·
Introduction to Methamphetamine/ Discovery and Early Usage/ World War II/ 1950’s to Today/ Meth’s affect on History/ How it is Made/ References
Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. (2000). History of the methamphetamine problem. Journal of Psychoactive Drugs. 32(2):137-41
Cunningham JK, Liu LM. (2003) Impacts of Federal ephedrine and pseudoephedrine regulations on methamphetamine-related hospital admissions. Addiction, 98, 1229-1237.
Doyle, D (2005). Hitler’s Medical Care. Journal of the Royal College of Physicians of Edinburgh 35: 75-82.
Iversen, L. (2006) Speed, Ecstasy, Ritalin, Oxford: OUP.
Meyer, U. (2005). Fritz hauschild (1908-1974) and drug research in the ‘German Democratic Republic’ (GDR). Pharmazie. 60(6): 468-72
Tamura, M. (1989). Japan: stimulant epidemics past and present. Bulletin on Narcotics 83-93. United Nations Office on Drugs and Crime.
Winslow BT, Voorhees KI, Pehl KA (2007). Methamphetamine abuse. American family physician 76 (8): 1169–1174.
Methamphetamine use dates to post-WWII era: drug little-known risk factor in early AIDS days. (2002). AIDS Alert 17(10): 125-127
http://www.chm.bris.ac.uk/motm/methamphetamine/methv.htm
http://en.wikipedia.org/wiki/Methamphetamine
http://www.emedicine.com/EMERG/topic859.htm
Categories: Methamphetamine
Tags: Jesse Birnbaum —










