Introduction – The Discovery – Locations where Methane is Found – Basic Science – How Methane Affects History – How History Affects Methane
Entries from April 2008
How History Affects Methane
April 30th, 2008 · Comments Off on How History Affects Methane
Categories: Methane
How Methane Affects History
April 30th, 2008 · 7 Comments
Introduction – The Discovery – Locations where Methane is Found – Basic Science – How Methane Affects History – How History Affects Methane
METHANE AND THE GREENHOUSE EFFECT
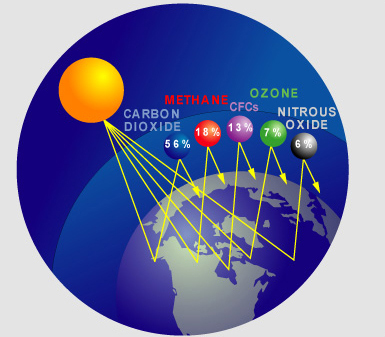 Methane (CH4) is a principal component of natural gas. It is also formed and released to the atmosphere by biological processes occurring in anaerobic environments. Once in the atmosphere, methane absorbs terrestrial infrared radiation that would otherwise escape to space. This property can contribute to the warming of the atmosphere, which is why methane is a greenhouse gas.
Methane (CH4) is a principal component of natural gas. It is also formed and released to the atmosphere by biological processes occurring in anaerobic environments. Once in the atmosphere, methane absorbs terrestrial infrared radiation that would otherwise escape to space. This property can contribute to the warming of the atmosphere, which is why methane is a greenhouse gas.
Categories: Methane
Basic Science
April 30th, 2008 · 1 Comment
Introduction – The Discovery – Locations where Methane is Found – Basic Science – How Methane Affects History – How History Affects Methane
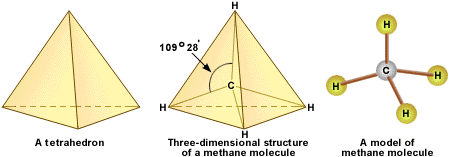
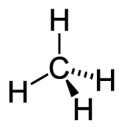
Structure
The strength of the carbon hydrogen covalent bond is among the strongest in all hydrocarbons. The carbon atom central to the methane molecule has 4 valence electrons and thus needs 4 more electrons from four hydrogen atoms to satisfy the octet rule. The hydrogen atoms have a 109 degree bond angle giving the molecule a tetrahedral geometry.
Physical Properties of Methane
Methane is an odorless, colorless, tasteless gas that is lighter than air. It is a non-polar molecule and is insoluble in water. It dissolves in non-polar solvents like alcohol.
Chemical Properties of Methane
When methane burns in the air it has a blue flame. In sufficient amounts of oxygen, methane burns to give carbon(CO2) dioxide and water(H2O). When it undergoes combustion is produces a great amount of heat and this is one of the reasons why it is used as a fuel source.

Cartoon demonstrating combustion properties of methane.
Categories: Methane
Locations Where Methane is Found
April 30th, 2008 · 4 Comments
Introduction – The Discovery – Locations where Methane is Found – Basic Science – How Methane Affects History – How History Affects Methane
NATURAL METHANE
Natural sources of methane include wetlands, gas hydrates, permafrost, termites, oceans, freshwater bodies, non-wetland soils, and other sources such as wildfires.
Wetlands
Natural wetlands are responsible for approximately 80% of global methane emissions from natural sources. Wetlands provide a habitat conducive to methane-producing bacteria that produce methane during the decomposition of organic material. These bacteria require environments with no oxygen and abundant organic matter, both of which are present in wetland conditions.
 Global emissions of termites are estimated to be about 11% of the global methane emissions from natural sources. Methane is produced in termites as part of their normal digestive process, and the amount generated varies among different species. Ultimately, emissions from termites depend largely on the population of these insects, which can also vary significantly among different regions of the world.
Global emissions of termites are estimated to be about 11% of the global methane emissions from natural sources. Methane is produced in termites as part of their normal digestive process, and the amount generated varies among different species. Ultimately, emissions from termites depend largely on the population of these insects, which can also vary significantly among different regions of the world.
Oceans
 Oceans are estimated to be responsible for about 8% of the global methane emissions from natural sources.The source of methane from oceans is not entirely clear, but two identified sources include the anaerobic digestion in marine zooplankton and fish, and also from methanogenisis in sediments and drainage areas along coastal regions.
Oceans are estimated to be responsible for about 8% of the global methane emissions from natural sources.The source of methane from oceans is not entirely clear, but two identified sources include the anaerobic digestion in marine zooplankton and fish, and also from methanogenisis in sediments and drainage areas along coastal regions.
Hydrates
 Global emissions from methane hydrates is estimated to account for approximately 5% of the global methane emissions from natural sources. Methane hydrates are solid deposits composed of cages of water molecules that contain molecules of methane. The solids can be found deep underground in polar regions and in ocean sediments of the outer continental margin throughout the world. Methane can be released from the hydrates with changes in temperature, pressure, salt concentrations, and other factors. Overall, the amount of methane stored in these hydrates globally is estimated to be very large with the potential for large releases of methane if there are significant breakdowns in the stability of the deposits.
Global emissions from methane hydrates is estimated to account for approximately 5% of the global methane emissions from natural sources. Methane hydrates are solid deposits composed of cages of water molecules that contain molecules of methane. The solids can be found deep underground in polar regions and in ocean sediments of the outer continental margin throughout the world. Methane can be released from the hydrates with changes in temperature, pressure, salt concentrations, and other factors. Overall, the amount of methane stored in these hydrates globally is estimated to be very large with the potential for large releases of methane if there are significant breakdowns in the stability of the deposits.
MAN-MADE METHANE
Human-related activities that produce methane include fossil fuel production, livestock industry, rice cultivation, biomass burning, and waste management. This is broken down into smaller categories above. These activities release significant quantities of methane to the atmosphere. It is estimated that 60% of global methane emissions are related to human-related activities.
Landfills
Landfills are the largest human-related source of methane in the U.S., accounting for 34% of all methane emissions. Methane is generated in landfills and open dumps as waste decomposes under anaerobic conditions (with no oxygen). The amount of methane created depends on the quantity and moisture content of the waste and the design and management practices at the site.
Natural Gas and Petroleum Systems
Methane is the primary component of natural gas. Methane losses occur during the production, processing, storage, transmission, and distribution of natural gas. Because gas is often found in conjunction with oil, the production, refinement, transportation, and storage of crude oil is also a source of methane emissions.
Coal Mining
Methane trapped in coal deposits is released during normal mining operations in both underground and surface mines. In addition, handling of the coal after mining results in methane emissions.
Livestock
Among domesticated livestock such as cattle, buffalo, sheep, goats, and camels, these animals produce significant amounts of methane as part of their normal digestive processes. In the rumen, or large fore-stomach of these animals, microbial fermentation converts feed into products that can be digested and utilized by the animal. This microbial fermentation process, referred to as enteric fermentation, produces methane as a by-product, which can be exhaled by the animal (cow farts if you will). (Methane is also produced in smaller quantities by the digestive processes of other animals, including humans, but emissions from these sources are insignificant.)
Wastewater Treatment
Wastewater from sewage is treated to remove soluble organic matter, suspended solids, pathogenic organisms, and chemical contaminants. These treatment processes can produce methane emissions if organic constituents in the wastewater are treated anaerobically (without oxygen) and if the methane produced is released to the atmosphere. In addition, the sludge produced from some treatment processes may be further biodegraded under anaerobic conditions, resulting in methane emissions.
Rice
 Methane is produced during flooded rice cultivation by the anaerobic (without oxygen) decomposition of organic matter in the soil. Flooded soils are ideal environments for methane production because of their high levels of organic substrates, oxygen-depleted conditions, and moisture. The level of emissions varies with soil conditions and production practices as well as climate. There have been cultivation practices that have shown promise for reducing methane emissions from rice cultivation.
Methane is produced during flooded rice cultivation by the anaerobic (without oxygen) decomposition of organic matter in the soil. Flooded soils are ideal environments for methane production because of their high levels of organic substrates, oxygen-depleted conditions, and moisture. The level of emissions varies with soil conditions and production practices as well as climate. There have been cultivation practices that have shown promise for reducing methane emissions from rice cultivation.
Categories: Methane
The Discovery
April 30th, 2008 · Comments Off on The Discovery
Introduction – The Discovery – Locations where Methane is Found – Basic Science – How Methane Affects History – How History Affects Methane
ALESSANDRO VOLTA
In his day Volta was an extremely well known scientist . While his primary field of expertise laid in the area of electricity(i.e- the “volt”), he also made significant discoveries in the field of chemistry. The first discovery of the natural production of methane gas is attributed to Alessandro Volta when he collected gas from the stirred up sediment from Lake Maggiore, Italy in 1778. He noticed large bubbles of gas coming to the surface and isolated them for further experimentation. His discovery was actually quite serendipitous in that he was merely on summer vacation, not actively attempting to make such a discovery.
Categories: Methane
Introduction
April 30th, 2008 · 4 Comments
Introduction – The Discovery – Locations where Methane is Found – Basic Science – How Methane Affects History – How History Affects Methane
WHAT IS METHANE?
Methane is a chemical compound with the molecular formula CH4. It is the main component in natural gas. Methane is considered the simplest of alkanes, compounds that consist only of hydrogen(H) and carbon(C) elements.
Categories: Methane
How Codeine Affects History
April 30th, 2008 · Comments Off on How Codeine Affects History
Background Information| Chemical Structure| How Codeine Works in the Body| Recreational Use| How Codeine Affects History
- Codeine is the first opiate derivative that has been made an over the counter drug.
- Despite its addictive nature, codeine is still a weak enough opiate that it’s the most prescribed drug in the world.
- Codeine is included in most medications that are needed to relieve pain and also has been added to many cancer and HIV medication which shows that the millions if not billions of people in the world depend on the drug.
- Despite the abuse of the drug recreationally, codeine still has helped the world of science for pain relief drugs.
Categories: Codeine
Recreational Use
April 30th, 2008 · 3 Comments
Background Information| Chemical Structure| How Codeine Works in the Body| Recreational Use| How Codeine Affects History
- Codeine can be used as a recreational drug, however it has much less abuse potential than some other opiates. When it is taken for recreational use, it is commonly referred to as “cilly” because of the way most people react to it.
- Codeine is the opioid which causes the most itching for a good percentage of users and its presence along with acetylcodeine in illicitly-produced heroin causes most of the itching associated with that drug.
- Codeine in conjunction with anti-nausea medication promethazine in the form of the syrup has become one of the most abused codeine preparations. Although there are various forms of this syrup varying in strengths, the “purple” version is highly publicized and is the most sought after. In this form, 60mg of codeine per liquid ounce is used which makes it the strongest of the codeine syrups. This “Purple Drank” is frequently referenced and praised in the southern rap and Houston-based hip-hop community where it is mixed with the soft drink Sprite.
Categories: Codeine
How Codeine Works in the Body
April 30th, 2008 · 3 Comments
Background Information| Chemical Structure| How Codeine Works in the Body| Recreational Use| How Codeine Affects History
- Codeine is a weak opiate agonist that provides relief from pain by blocking the pain signals that are being sent from the brain to affected areas of the body.
- It works on the Central Nervous System (CNS) to inhibit these pain signals. Because the transmission of the pain signals has been impaired, less pain is felt, even if the original source of the pain remains.
- Codeine mimics the effects of endorphins, which are naturally occurring painkilling chemicals produced by the body.
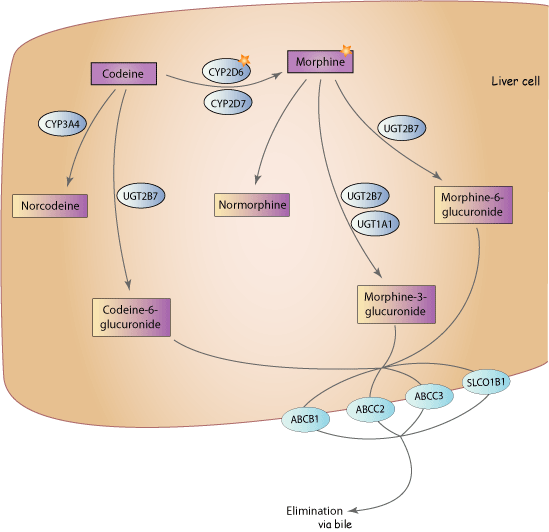
- To experience the painkilling properties of codeine the body has to convert it into morphine. Codeine is absorbed by the gastrointestinal tract, becoming quickly transported to various tissues throughout the body.
- Codeine does not accumulate in body tissues because it is metabolized by the liver and its metabolic products are excreted by the kidneys. The process by which codeine is metabolized is known as glucuronidation. Through O-demethylation the codeine is converted into morphine and through N-demethylation it becomes norcodeine.
- The metabolism rate is approximately 30 mg of codeine in an hour and about 90% of the drug will be excreted from the body within a day. In most people, only about 10% of codeine is transformed into morphine.
Categories: Codeine
Chemical Structure
April 30th, 2008 · Comments Off on Chemical Structure
Background Information| Chemical Structure| How Codeine Works in the Body| Recreational Use| How Codeine Affects History
- The chemical formula for codeine is C18H21NO3
- The chemical name for the drug is expoxy-3methoxy-17-methylmorphian-6-ol.
- The alternative names for codeine are methylmorphine and morphine monomethyl ether.
- Boiling Point: 250C (480.F) at 22mm
- Melting Point: 154-156.C (309.2-312.8.F)
- The drug is slightly soluble in water and freely soluble in alcohol
Categories: Codeine