Introduction – The Discovery – Locations where Methane is Found – Basic Science – How Methane Affects History – How History Affects Methane
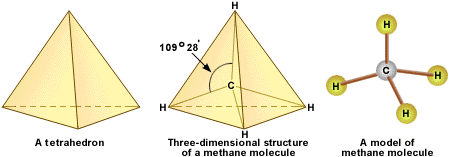
Structure
The strength of the carbon hydrogen covalent bond is among the strongest in all hydrocarbons. The carbon atom central to the methane molecule has 4 valence electrons and thus needs 4 more electrons from four hydrogen atoms to satisfy the octet rule. The hydrogen atoms have a 109 degree bond angle giving the molecule a tetrahedral geometry.
Physical Properties of Methane
Methane is an odorless, colorless, tasteless gas that is lighter than air. It is a non-polar molecule and is insoluble in water. It dissolves in non-polar solvents like alcohol.
Chemical Properties of Methane
When methane burns in the air it has a blue flame. In sufficient amounts of oxygen, methane burns to give carbon(CO2) dioxide and water(H2O). When it undergoes combustion is produces a great amount of heat and this is one of the reasons why it is used as a fuel source.

Cartoon demonstrating combustion properties of methane.