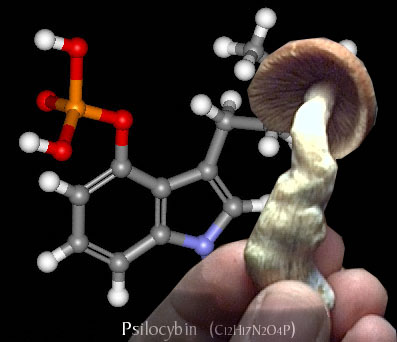
Introduction to Psilocybin – Effects of Psilocybin – History of Psilocybin
Not long after the initial discovery of magic mushrooms and their effects, that these hallucinogenic fungi before the chemistry involved with these experiences and the drug was examined under a microscope.
· The main type, Psilocybe genus, of mushrooms was found to contain the active ingredients of psilocybin and psilocin.
· After examination a variety of four and five hydroxylated N-methyeltryptamines were isolated from the mushrooms.
· The two compounds, psilocybin and psilocin, are equally psychoactive. Colorless psilocybin can be rapidly oxidized to reveal a blue colored product, which is one of its most common field identification features.
Molecules
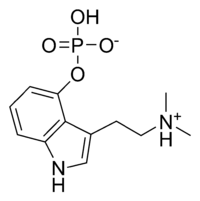
· One molecule of psilocybin (O-phosphoryl-4-hydroxy-N) can be broken down into one molecule of psilocin (4-hydroxy-N, N-dimethyl-tryptamine).
· Psilocybin is quickly dephosphorylated to its phosphate ester, psilocin, by alkaline phosphatase, an enzyme present both in the brush border of the intestines and in the liver.
· This removes the phosphate group from psilocybin then leaving, psilocin to be passed along through the bloodstream.
· This breakdown occurs when the mushroom is orally ingested
· Within thirty minutes the toxins from the drug will have equilibrated, although it accumulates in the liver and adrenal glands.
· This accumulation in the liver and adrenal glands continues for up forty right hours, which could be a very good explanation for the prolonged euphoric sensation that is experienced by many after an ingestion of mushrooms.
· It is interesting that monoamine oxidase, the enzyme normally responsible for the metabolism and degradation of other biogenic amines, such as serotonin, plays almost no role in the elimination of psilocin.
· Structural similarities to the biogenic amines responsible for neurotransmission, such as dopamine and serotonin, suggest that the effects of psilocin are passed through the same pathways, perhaps by “antagonizing” serotonin
· It has also been considered that psilocybin and psilocin, “like other hallucinogenic drugs analogous to serotonin, have serotonin-like and anti-serotonin activities. Possibly their effects on the mind could be related to these actions.”
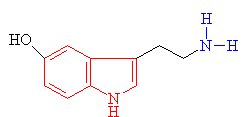
| Serotonin The indole ring is shown in red and the amine group in blue |
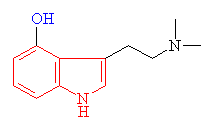
Psilocin The hydrolysed form of psilocybin with the change shown in blue |
· As you can see above the two molecules both contain indole rings, which are a 6- membered benzene ring fused to a 5-membered ring containing nitrogen.
· The only difference is the addition of methyl groups. This makes the molecule more lipophilic, soluble in fats, in turn, better able to penetrate the fatty membranes that protect nerves and nerve endings.
· This allows the molecules to easily penetrate the central nervous system, making them more potent.
· Psilocybin has this indole ring, with a phosphoric acid group attached to it. In the body the phosphoric acid group is oxidized to the hydroxyl compound, psilocin, as previously discussed