Entries Tagged as 'Calcium Carbonate'
April 28th, 2008 · 1 Comment
Introduction to Concrete | Analysis of CaCO3 | Concrete Affects History | Industrial Development | Calcium Carbonate Accomplishments | Damage | History Affects CaCO3: Future of Concrete
Future of Concrete
- What kind of environmental damages are the result of limestone and calcium carbonate quarrying?
- Is it possible that the world can run out of concrete, with the copious amount of rock and aggregate?
The answer: Yes!
Many companies today are engaging in concrete recycling programs, which can keep the industry going strong without the detrimental factors involved with collecting rocks and chemicals needed for cement production, and the limited amount of some earthly materials, such as essential aggregages.
Another major aspect to the future of concrete is High Performance Concretes where special mixtures are invented using new technology that enhance the quality and versatility of the amazing substance. Everyday, scientists find new chemicals that supplant concrete’s strength, drying time, and stamina.
With Programs like these, The Future looks long and bright for CaCO3.

Pure Calcium Carbonate: The stuff that started it all!
THANK YOU FOR READING,
Matthew Schmiemann
[Read more →]
Categories: Calcium Carbonate
Introduction to Concrete | Analysis of CaCO3 | Concrete Affects History | Industrial Development | Calcium Carbonate Accomplishments | Damage | History Affects CaCO3: Future of Concrete
The Ugly Side of CaCO3
- Over the years, concrete has been responsible for a large amount of mishaps, due to the damage that can accumulate with its usage, such as cracks, fires, and chemical corrosion. Disaster can occur once a significant amount of damage is done. In this way, calcium carbonate affects history in a negative way, as it can destroy just as much as it can create.
- Bacterial Damage – Bacteria can produce hydrogen sulfide, which oxidizes into the corrosive sulfuric acid, harming the surface of concrete. However, calcium carbonate in limestone can make the concrete more acid resistant.
- Chemical Damage – The over use of substances that contain too much acid, such as sulfates, chlorates, and certain carbonates increase the pH of the concrete and can lead to cracking and dissolving. This is a serious issue with construction, as too much damage can cause a building to collapse.
- Sea Water Damage – A particularly harmful effect on concrete structures is the erosion that occurs when it comes into contact with running water, such as bridges, where over the years, a bridge can collapse because of the amount of undersea damage sustained. Damage can be done to cars, people could get hurt, and traffic would be insane in an event such as this.
The Ugly sight of what can happen when concrete suspensions fail due to structural damage, built up over time.

Conclusion: Damage leads to disasters, disasters greatly affect history.
http://img.timeinc.net/time/photoessays/2007/minneapolis_bridge/minneapolis_bridge_01.jpg
http://en.wikipedia.org/wiki/Concrete
[Read more →]
Categories: Calcium Carbonate
Introduction to Concrete | Analysis of CaCO3 | Concrete Affects History | Industrial Development | Calcium Carbonate Accomplishments | Damage | History Affects CaCO3: Future of Concrete
Some Amazing CaCO3 Accomplishments
 Mount Rushmore, near Keystone, ND, shows the great facades of our Presidents, comprised of almost complete granite, which is a rock that includes calcium carbonate deposits. The strong molecular structure of the rock alled for dynamite to blast through the stone cliff.
Mount Rushmore, near Keystone, ND, shows the great facades of our Presidents, comprised of almost complete granite, which is a rock that includes calcium carbonate deposits. The strong molecular structure of the rock alled for dynamite to blast through the stone cliff.

In 1984, The Statue of Liberty was closed so that a $62 million renovation could be performed. Concrete was a part of that renovation because in the places where the paint corroded the internal copper skeleton, and where steel was inappropriate to use, concrete was used to reinforce the areas. So in a way, CaCO3 was a part of the reason why the Lady is still standing today.
The Hoover Dam, the world’s largest complete concrete structure, is a $49 million dollar accomplishment that allows passage from Arizona to Nevada, holds back millions of gallons of water, and generates 2,080 megawatts of electrical power to hundreds of homes. Up to 10 million people visit each year. CaCO3 saves the day again.
Limestone, Limestone, Limestone
 Even our own buildings on Dickinson College campus are lined with beautiful limestone, which is Calcium Carbonate.
Even our own buildings on Dickinson College campus are lined with beautiful limestone, which is Calcium Carbonate.
[Read more →]
Categories: Calcium Carbonate
April 28th, 2008 · Comments Off on Industrial Development
Introduction to Concrete | Analysis of CaCO3 | Concrete Affects History | Industrial Development | Calcium Carbonate Accomplishments | Damage | History Affects CaCO3: Future of Concrete
Calcium Carbonate has shaped history by establishing a World-Wide Economic Industry
- The past half-decade saw a global expansion of 633 million tons.
- Almost 70%, or 440 million tons of this comes from East Asia alone.
- 76 million tons come from other Asian countries.
- 7 million tons from Central and South America
- 44 million tons from Africa/Middle East
- 48 million tons from Europe
- 20 million tons from North America.
 A Chinese International Cement Industry Exhibition
A Chinese International Cement Industry Exhibition
Cement trade
- The world cement trade has risen almost continuously over the past 35 years, advancing from 20 million tons in 1970, to 71 million tons by 1990, and to 155 million tons last year.
- Current cement consumption closely mirrors that of production, emphacizing the relatively low level of international cement trade volumes relative to world demand. (Good thing we won’t be running out of it anytime soon.)
Hawaii’s Volcanic Ash
The Hawaiian Islands have been coveted and industrialized for its supplies of volcanic ash. The ash is an important aggregate mixture with the CaCO3 limestone in certain mixtures. This has brough much wealth to the tiny state, and although the pulvurization of the islands has harmful environmental effects, the concrete industry gives it something else to export besides sugarcane.
Staple of Modern Construction
The versitility, strength, and overall availablility of Calcium Carbonate and other earthly compounds make concrete the most widely used building material all around the world. Take a look around you, and see just how much of where you live, work, and play is dictated by the use of concrete to suspend you, your car, and everything else. Truely, CaCO3 has had a profound effect on our world, our industries, and how we have come to know them today.


(above, left: The New Rector Science Complex, under construction on the Dickinson College campus, using an internal concrete support structure, Carlisle, PA.) circ. 2007
(above, right: The Tallest Precast Concrete Building West of the Mississippi, The Paramount Building is Topped Off in San Francisco, CA. It was one of the first to employ methods that Dickinson is doing right now.) circ. 2001
Specific Industrial Uses of Calcium Carbonate
- Additive to calcium supplement health tablets and anacids.
- Additive to liquid products to create a paste, such as in toothpaste.
- Ingredient in babies’ diapers to create its microporous film lining.
- Pure CaCO3 can be marketed as blackboard/sidewalk chalk.
- Used as a food additive in calcium shakes, soy milk, and baby formula.
- MAKING POKER CHIPS!!! YAY FOR CACO3 GAMBLING!!!
http://www.cementchina.net/news/shownews.asp?id=1570
http://findarticles.com/p/articles/mi_m0NSY/is_6_24/ai_n16497840
www.dickinson.edu/…/august/images/NSC-1712.jpg
http://www.lehighsw.com/projects/proj.htm
http://en.wikipedia.org/wiki/Calcium_carbonate
[Read more →]
Categories: Calcium Carbonate
April 28th, 2008 · Comments Off on Concrete Affects History
Introduction to Concrete | Analysis of CaCO3 | Concrete Affects History | Industrial Development | Calcium Carbonate Accomplishments | Damage | History Affects CaCO3: Future of Concrete
- The earliest records of primitive concrete being made date back to 5600 BC in modern day Serbia, with a hut made of a red lime, sand, and gravel mixture. (This was before even the Sumerian Civilization began in Mesopotamia.)
-
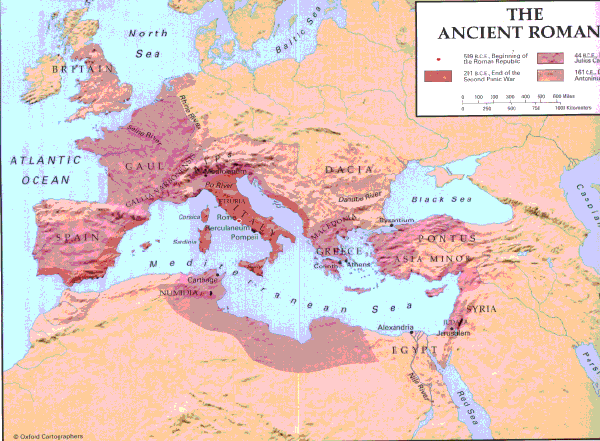
The Romans have Calcium Carbonate to thank for their Empire (approx 700 BC-100 BC), because their great cites were supplied with fresh water from concrete aquaducts, made from a quicklime, pozzolanic ash, and pumice mixture, along with some additives like Horse hair and blood.
(A Roman aquaduct remains in France.) 
-
From the 1850’s on, after Joeseph Aspdin patented his Portland Cement, the perfected mixture found its way to making roads, bridges, tunnels and dams in all areas of the world. These structures revolutionized many countries as they could now navigate to new areas and trade with areas that before were unreachable.
 (Even if the road is not made of cement, chances are that the structures suspending it are. Yay CaCO3!!)
(Even if the road is not made of cement, chances are that the structures suspending it are. Yay CaCO3!!)
http://www.kuhlman-corp.com/HistoryCon.html
[Read more →]
Categories: Calcium Carbonate
Introduction to Concrete | Analysis of CaCO3 | Concrete Affects History | Industrial Development | Calcium Carbonate Accomplishments | Damage | History Affects CaCO3: Future of Concrete
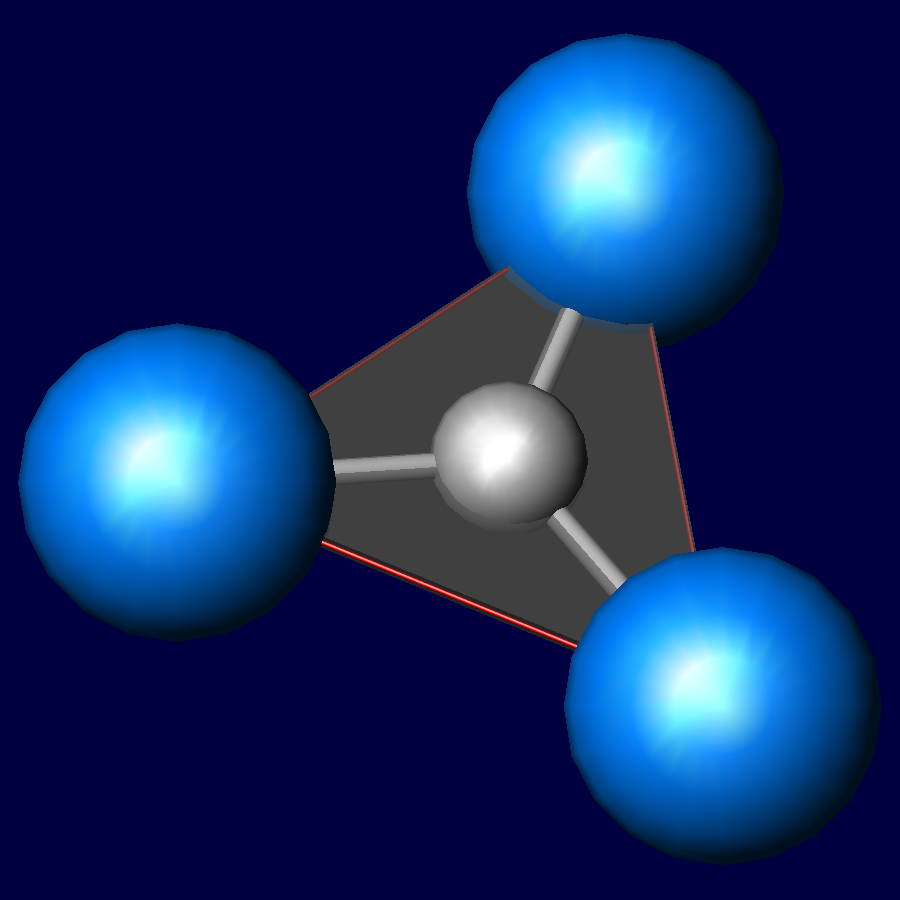
The Carbonate Molecule CO3: A Trigonal Planer Arrangement
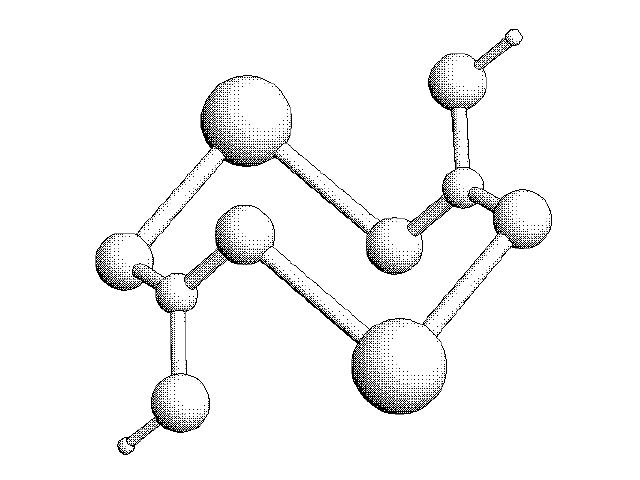
3D Sketch of two CO3 Molecules interacting with Calcium Ions: The CaCO3 Molecule. They form a Semi-“pleated sheet” crystalline structure.
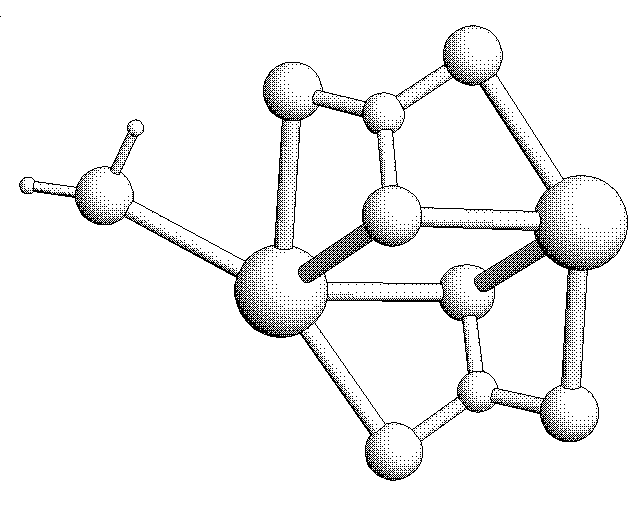
With the introduction of water (H2O) molecules, the structure of the CaCO3 molecules change. The molecules form a permeable “cage” that can move as liquid, but solidify as rock under heat and pressure.
- When the calcium carbonates first come into contact with water, a reaction occurs in which calcium ions are formed and the water molecules are broken down to form hydroxide ions. It is this bond breaking that produces heat. Calcium hydroxide is not very soluble so it is soon saturated and forms a solid.
- As long as water is in contact with the cement, these reactions continue but they get slower and slower, and can take several years to reach full strength.
- As the solid is formed, however, there is less and less space between the grains of aggregate and carbonate for the water to move around and reach un-reacted cement. This means that the reaction will slow down as the pores between the aggregate get smaller.
(until you become this guy!!) 
http://chemistry.suite101.com/article.cfm/the_chemistry_of_concrete
http://www1.elsevier.com/homepage/saa/eccc3/paper54/paper54.html
[Read more →]
Categories: Calcium Carbonate
Introduction to Concrete | Analysis of CaCO3 | Concrete Affects History | Industrial Development | Calcium Carbonate Accomplishments | Damage | History Affects CaCO3: Future of Concrete
 ——————->
——————-> 
In the world of construction, one material is used above all others: concrete. Concrete is absolutely indespensible in modern society’s fascination with new roads, buildings and other constructions. One industry expert has gone as far as to say that now “concrete IS chemistry.” This is due to the increasing development of admixtures which chemically affect certain properties of concrete. -Simon Davies The Chemistry of Concrete
-
The three basic ingredients of concrete are aggregate, cement, water. Cement is the fixture that binds the ingredients together, water gives the concrete viscocity in order to be molded and react with the ingredients, and the aggregates are what adds bulk to the concrete, but are not involved in the chemical processes.
-
Cement, the building block of concrete is composed of innumerable, natural occuring elements, earthly compounds, and alkali metals: among them is Calcuim Carbonate. The cement mixture is created by crushing up clay and limestone together and roasting it in a kiln.

The formed powder typically has a similar chemistry to the following:
75% Di, Tri- calcium silicate
10% Tetracalcium aluminoferrite
10% Tricalcium aluminate
10% Gypsum or Hydrated calcium suphate
- Calcium Carbonate serves as a general term referring to the limestone base that is quarried from mines in order to complete the mixture, and is especially important.
- Some other common materials found in finished Concrete are chalks, marbles, oyster shell, shale, clay, slags, fly ash, bauxite, alumina process waste, and granite, silica sand, iron ores, blast furnace flue dusts, pyrite clinker, and mill scale. CaCO3 is present in most. -Charles Kubach, Mineral Processing Engineer, Sepor Inc.
http://chemistry.suite101.com/article.cfm/the_chemistry_of_concrete
http://www.mine-engineer.com/mining/cement.htm
[Read more →]
Categories: Calcium Carbonate











 (CaCO3 remains as a reminder of the great power of Ancient Egypt during 3000 BC.)
(CaCO3 remains as a reminder of the great power of Ancient Egypt during 3000 BC.)






