Introduction to Concrete | Analysis of CaCO3 | Concrete Affects History | Industrial Development | Calcium Carbonate Accomplishments | Damage | History Affects CaCO3: Future of Concrete
-
The properites and abilities of concrete are derived from the molecular stucture of CaCO3 and of other molecules like it.
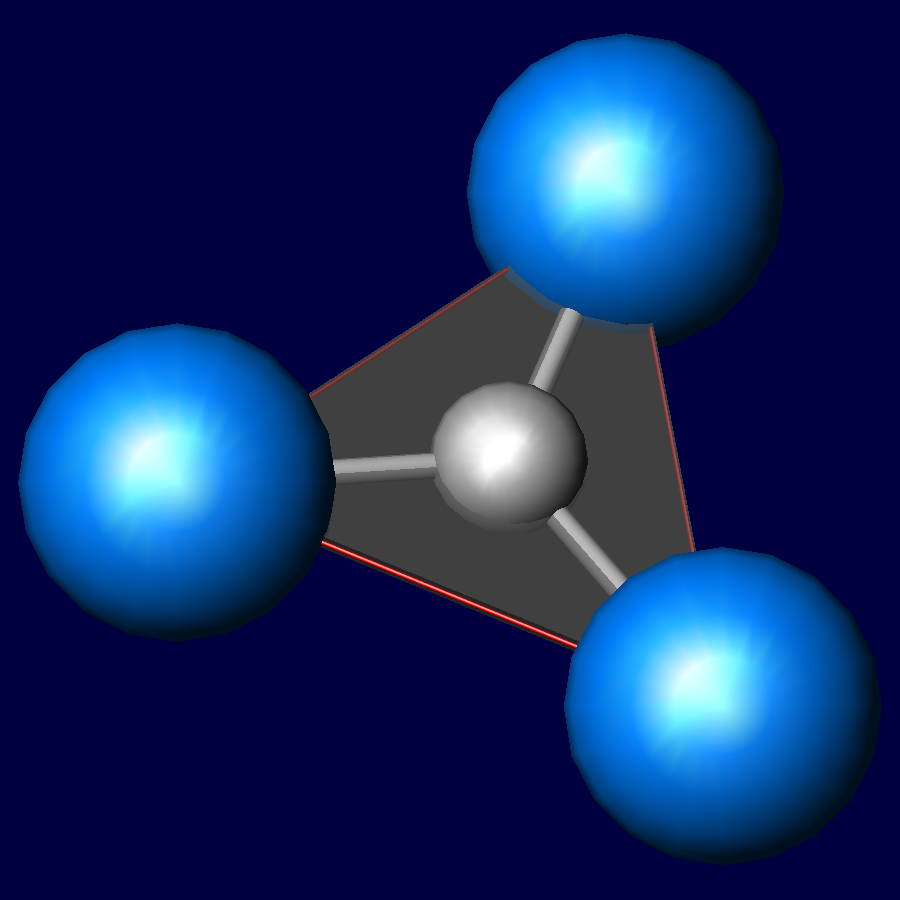
The Carbonate Molecule CO3: A Trigonal Planer Arrangement
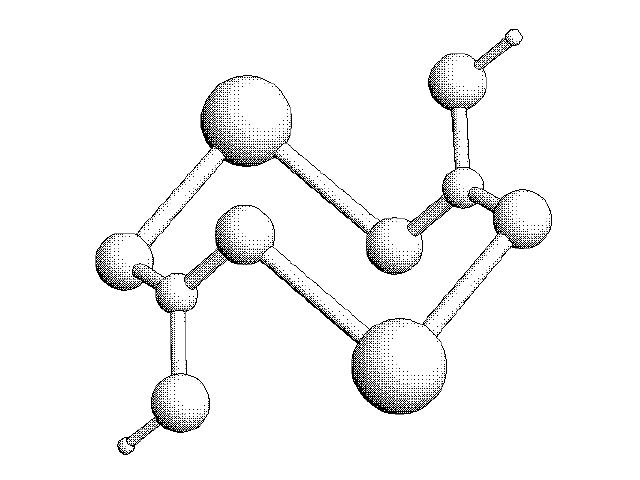
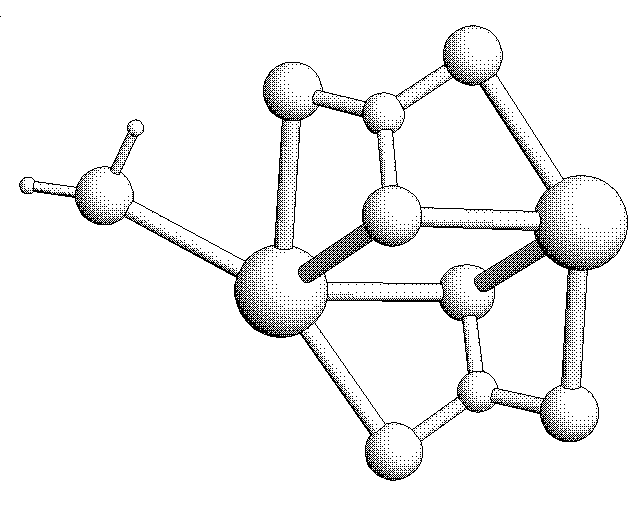
3D Sketch of two CO3 Molecules interacting with Calcium Ions: The CaCO3 Molecule.
With the introduction of water (H2O) molecules, the structure of the CaCO3 molecules change. The molecules form a permeable “cage” that can move as liquid, but solidify as rock under heat and pressure.
- When the calcium carbonates first come into contact with water, a reaction occurs in which calcium ions are formed and the water molecules are broken down to form hydroxide ions. It is this bond breaking that produces heat. Calcium hydroxide is not very soluble so it is soon saturated and forms a solid.
- As long as water is in contact with the cement, these reactions continue but they get slower and slower, and can take several years to reach full strength.
- As the solid is formed, however, there is less and less space between the grains of aggregate and carbonate for the water to move around and reach un-reacted cement. This means that the reaction will slow down as the pores between the aggregate get smaller.
http://chemistry.suite101.com/article.cfm/the_chemistry_of_concrete
http://www1.elsevier.com/homepage/saa/eccc3/paper54/paper54.html