Entries Tagged as 'Ozone'
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
Ozone is a very potent oxidizing agent, which can turn an olefin into a ketone, a carboxylic acid or an aldehyde. It is also used as a commercial bleaching agent, effective on organic compounds. Ozone is also put in drinking water so it can act as a germicide and sterilize the water. While in the water it can also take away unfavorable tastes and smells. Ozone is used in whiskey distilleries as a way to destroy the empyreumatic taste of whiskey. It is useful as a decomposition agent. It can decompose dangerous organic and inorganic chemical wastes such as phenols, pesticides, detergents, and cyanides. Ozone can also help precipitate out unwanted heavy metal ions and hydroxides, like iron and manganese. With ozone, one can control not only the formation of slime, but also the odor in sewage treatment and in manufacturing industries.
[Read more →]
Categories: Ozone
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
In the late 1920s, there was a need for a safe refrigerant. Thomas Midgley, who was working for General Motors at the time, found the answer in chlorofluorocarbons, or CFCs. This seemed to be the perfect response because CFCs are inert in the troposphere. So they were used in air conditioning, aerosols and other appliances. However, in 1974 Mario Molina and Sherry Rowland put forth the hypothesis that the decline in concentration of the ozone layer was due to the CFCs. This process of man-made destruction of ozone happens because CFCs are long lasting, inert molecules that can accumulate. Winds carry the CFCs up to the stratosphere, where a UV ray breaks off a chlorine from the CFC, which is highly reactive and takes one of the oxygen atoms in ozone and bonds to that. Then the oxygen atom bond to another oxygen atom and the chlorine is free to repeat the process. A single atom of chlorine can kill tens of thousands of ozone molecules before it is removed. The CFCs travel to Antarctica because of wind patterns and accumulate there, causing the ozone hole. The hole is also due to chlorine in reservoir species, ClONO2 and HCl, where they get into polar stratospheric clouds and can go reactions which release the chlorine atom. Because natural ozone formation and destruction needs the Sun’s rays, the destruction of the ozone layer can have an accumulation effect if the levels are never recovered between winters. In the decade following Molina and Rowland’s findings, industry battled science about the production of CFCs. In the early 1980s evidence proving their point mounted. With data going back to 1957, there was a clear decline in ozone concentration and really picked up in the early 1980s. Also, findings showed that in places with decreased reservoir species had an abundance of active chlorine compounds and a decreased ozone level. So, in 1987 the Montreal Protocol was held and they decided to cut CFC production in half. This was a monumental occasion because it was the first international attempt to prevent ozone destruction. Ozone destroying chemicals dropped ninety-five percent since the late 1980s, and CFCs were eventually banned in the United States.
History also effects ozone in the troposphere. With the mass production of cars in the early twentieth century came pollution. The combustion of fossil fuels in automobiles creates nitrogen dioxide, which breaks apart because of UV light into a nitrogen oxide radical and oxygen radical. The oxygen radical then bonds with an oxygen molecule, creating ozone.

�
[Read more →]
Categories: Ozone
April 23rd, 2008 · 1 Comment
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
The Ozone layer is located around twelve to twenty five kilometers, or eight to fifteen miles, above the Earth’s surface. The layer protects us from the harmful UV rays by absorption. If the UV rays would get to the Earth’s surface, then life as we know it today would not exist. Also, if the UV rays can get to the surface now, it will have a severely negative impact on DNA and RNA. One billion tonnes of ozone is naturally formed and destroyed a year. Ozone is formed naturally when a UV ray breaks the bond of oxygen-to-oxygen, leaving two atoms. Then, with the help of another atom that is bonded to another oxygen molecule, the oxygen atom forms a bond to the oxygen molecule. It is naturally destroyed when a UV ray breaks one of the bonds in ozone, leaving an oxygen molecule and an oxygen atom. Each of these processes happen at the same rate, leaving a constant amount of ozone in the stratosphere.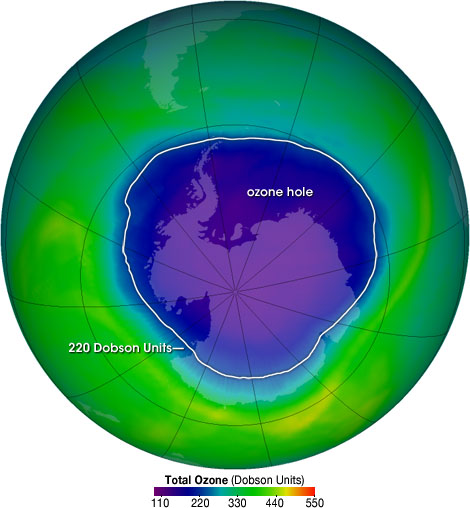 �
�
[Read more →]
Categories: Ozone
April 23rd, 2008 · Comments Off on Ozone in the Troposphere
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
Lower troposphere ozone impacts human health, crops, forests and natural ecosystems, so there is effort to keep the ozone levels at a minimum. Both industrialized and developing countries have been found to have potentially harmful levels of ozone. Since the 1970’s there has been a constant increase in ozone concentration, raising concerns. Ozone irritates eyes and nasal passages, and people with asthma or heart disease are especially susceptible to the negative effects. If one is exposed to ozone levels at .3 ppm for one to two hours, fatigue and respiratory difficulties result. Ozone is also very poisonous to plants, especially leafy ones like tobacco and tomatoes. It reduces photosynthesis, which causes leaves to turn yellow.

�
[Read more →]
Categories: Ozone
April 23rd, 2008 · Comments Off on Ozone History
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
After a lightening strike, there is a peculiar odor that man has been aware of for centuries. It has been mentioned works such as The Illiad and The Odyssey. In 1785 Martin van Marum studied the passing of an electrical spark through diatomic oxygen and noticed a sweet smell. He attributed the smell to the electricity, calling it the “electrical odor.” It was not until Christian Schöbein studied the odor that he realized it did not come from the electricity, but was a different molecule. He named it ozone after the Greek word ozein, which means to smell. Schöbein is also the founder of guncotton, when he wiped nitric acid with a cotton cloth in his kitchen. The actual structure was not determined until T. Sterry Hunt hypothesized it was three oxygen atoms. The fact that ozone is so widespread was not realized until 1850. Gordon Dobson made the first tool to measure ozone and surface measurements began in the 1860s. The discovery of the ozone layer in the stratosphere did not come until the early Twentieth century.

�
[Read more →]
Categories: Ozone
April 23rd, 2008 · Comments Off on Ozone Introduction
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
Ozone is a molecule comprised of three oxygen atoms. It is bent, like water, with one double bond and one single bond.
[Read more →]
Categories: Ozone


 �
�
