DIAZEPAM
Introduction to Diazepam History of Diazepam Chemistry
Dosage/Dependence/Toxicity Forms and Names


DIAZEPAM
Introduction to Diazepam History of Diazepam Chemistry
Dosage/Dependence/Toxicity Forms and Names

Categories: Diazepam
Forms
Diazepam can be prescribed and taken in oral, rectal, inhalation, and injection forms. Injections are used for uses with children.
Names
Trade names for diazepam include Valium (most common in America), Novodipam, and Valpam.
Recreational Use
Self-medication in frequent with diazepam because of the strong dependence formed. *In Sweden, over 25% of DUIs were due to diazepam.*
Categories: Diazepam
Dosage
Typical dosages for healthy adults range from 2 mg per dose to 10 mg per dose taken 2 to 4 times per day, depending on such factors as body weight and condition being treated. However, dosage is usually increased with time due to a tolerance buildup.
Dependence
Diazepam as with other benzodiazepine drugs can cause physical dependence, addiction and what is known as the benzodiazepine withdrawal syndrome . Withdrawal from diazepam or other benzodiazepines often leads to withdrawal symptoms which are similar to those seen during alcohol and barbituate withdrawal. The higher the dose and the longer the drug is taken for the greater the risk of experiencing unpleasant withdrawal symptoms. Withdrawal symptoms can occur from standard dosages and also after short term use.
Toxicity
Although not usually fatal when taken alone, a diazepam overdose is considered a medical emergency and generally requires the immediate attention of medical personnel. The antidote for an overdose of diazepam (or any other benzodiazepine) is flumazenil (Anexate). This drug is only used in cases with severe respiratory depression or cardiovascular complications.
Symptoms of an overdose of diazepam include coma, hypo tension, and mental confusion along with impaired motor functions.
Categories: Diazepam
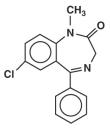
C16H 13ClN2O
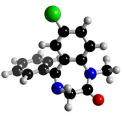
Diazepam is a white, light yellow crystalline powder. It does not have a smell and tastes slightly bitter. The melting point of diazepam is between 130-134 degrees C. It is soluble in Chloroform, but in water it is almost totally insoluble. Also, diazepam in neutral with a pH level of 7.
Categories: Diazepam
Invented by Dr. Leo Sternbach of Hoffman-La Roche and approved for use in 1963, Diazepam is two and a half times more potent than its predecessor, chlordiazepoxide, which it quickly surpassed in terms of sales.Diazepam was the top-selling pharmaceutical in the United States from 1969 to 1982, with peak sales in 1978 of 2.3 billion tablets.
Diazepam is also found in nature. Several plants, such as potato and wheat, contain trace amounts of naturally occurring diazepam and other benzodiazepines.
Diazepam is a core medicine in the World Health Organization’s “Essential Drugs List”, which lists the minimum medical needs for a health care system.
Diazepam was originally designed for the treatment of anxiety disorders and is also used for the treatment of agitation, tremors, delirium, seizures, and hallucinations resulting from alchohol withdrawl. It is used for the treatment of seizures and relief of muscle spasms in some neurological diseases including stiff-person syndrome.
Categories: Diazepam

Diazepam is in a group of drugs called benzodiazepines Diazepam affects chemicals in the brain that may become unbalanced and cause anxiety.
Diazepam is used for the management of anxiety disorders or for the short-term relief of symptoms of anxiety. It may also be used to relieve agitation, shakiness, and hallucinations during alcohol withdrawal and to relieve certain types of muscle spasms. It may also be used to treat seizures, insomnia, and other conditions as determined by your doctor.
Categories: Diazepam