April 29th, 2008 · Comments Off on Ammonia Affects History
Introduction | Chemical and Physical Properties | Synthesis of Ammonia: The Haber-Bosch Process | A Fertilizer | Ammonia Affects History | Other Uses
- Germany and World War I
- During this time, Chile was the biggest exporter of nitrates to Germany. Germany used these nitrates to produce the supply of their munitions during the war
- A naval blockade was formed that prevented Germany from getting its supply of nitrates from Chile. Unless something was done quickly, Germany would run of munitions to continue to fight the war
- Rescue came in the form of ammonia and the Haber-Bosch Process. Germany devised a way using the Haber-Bosch process to convert ammonia into nitric acid and nitrates. It proved to be incredibly beneficial to Germany who now had a much more efficient way of producing munitions
- Many believe that without the Haber-Bosch Process, Germany would have almost certainly run out of explosives in 1916, ending the war three years earlier than it actually did
- Fertilizer and World Population
- After World War II, there was a rising demand for nitrogen fertilizers. Thus, the production of ammonia shifted to the purpose of producing it for fertilizers
- The efficiently of crop production increased exponentially
- The Haber-Bosh synthesis of ammonia removed the limits on nitrogen supply that had constrained traditional agriculture
- This allowed for a large population increase around the world
- Raised standard of living in Third World Countries
- Changed diets
- Malnutrition was greatly decreased.
- The use of ammonia-based fertilizers also made it possible to grow crops in places that normally could not grow crops
- About 85% of all nitrogen in food proteins available for human consumption comes directly in plant foods from the world’s cropland and synthetic nitrogen fertilizers provide about half of the nutrient in those harvests crops
- Therefore, more than 40% of the world’s protein supply comes the Haber-Bosch synthesis of ammonia
- In other words, the synthesis of ammonia is responsible for feeding 2.2 billion people
[Read more →]
Categories: Ammonia
April 29th, 2008 · Comments Off on Other Uses
Introduction | Chemical and Physical Properties | Synthesis of Ammonia: The Haber-Bosch Process | A Fertilizer | Ammonia Affects History | Other Uses
- Synthetic fibers such as nylon and rayon
- Dying of cotton, wool, and silk
- Production of nitric acid in a method known as the Ostwald process
- Household cleansing agent
- Used in cigarettes during the 1960s
[Read more →]
Categories: Ammonia
April 29th, 2008 · Comments Off on A Fertilizer
Introduction | Chemical and Physical Properties | Synthesis of Ammonia: The Haber-Bosch Process | A Fertilizer | Ammonia Affects History | Other Uses
- The main use of ammonia is in fertilizers. Fertilizers are used as a substance added to soil in order to improve the growth and yield of plants. All plants rely on nutrients in the soil in order to survive, most importantly nitrogen. However, as the plants grow they use up those nutrients in the soil. Thus, something is needed in order to replenish those vital nutrients. Fertilizers replace the chemical components that are taken from the soil by growing plants. The replenishing of nutrients in the soil by fertilizers make the plans grow more efficiently.
- Modern-day research into fertilizers began in the early 1600s. Scientists began to discover the chemical needs of plants and thus began to improve fertilizers. Organic chemists Justus con Liebig discovered that plants needed mineral elements such as nitrogen and phosphorous in order to grow. Subsequently, the first patent was issued to John Lawes for his method of producing a form of phosphate that was effective as a fertilizer. Lawes’ patent is often said to have originated the fertilizer industry. The fertilizer industry then experienced its most significant growth after World War I when ammonia facilities that were producing explosives for the war were converted to the production of fertilizers.
- It was this post-war era that saw the growth of fully integrated factories deigned to produce fertilizers. It was quickly realized that ammonia would be a good source for these fertilizers because it could be synthesized from inexpensive raw materials.
[Read more →]
Categories: Ammonia
April 29th, 2008 · Comments Off on Chemical and Physical Properties
Introduction | Chemical and Physical Properties | Synthesis of Ammonia: The Haber-Bosch Process | A Fertilizer | Ammonia Affects History | Other Uses
- Ammonia is a colorless gas with a strong, pungent odor
- It is a chemical consisting of one nitrogen atom and three hydrogen atoms (NH3)
- It has a trigonal pyramidal shape with the nitrogen atom at its center
- The nitrogen atom has a lone electron pair
Ball-and-Stick Model of Ammonia:
Molecular Structure of Ammonia:
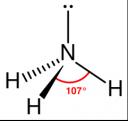
[Read more →]
Categories: Ammonia
April 29th, 2008 · 1 Comment
Introduction | Chemical and Physical Properties | Synthesis of Ammonia: The Haber-Bosch Process | A Fertilizer | Ammonia Affects History | Other Uses
Many would be surprised to hear that one of the most significant and important contributions to the well being of humankind over the past two hundred years has been the industrial synthesis of ammonia. Little do people know that their very existence on this earth today may possibly be a result of ammonia.
Since nitrogen is the crucial element for all living organisms and ammonia is a great source of nitrogen, the synthesis of ammonia was greatly sought after. When its synthesis was finally discovered in the early 1900s, it gave birth to the fertilizer industry.
Fertilizers greatly increased crop yields around the world resulting in massive population increases during the 20th century. In the 1990s, synthetic fertilizers were responsible for producing 40% of the world’s food. That means that without ammonia more than 2.2 billion people would not be on this planet today!
[Read more →]
Categories: Ammonia


