Introduction / DDT molecule / Malaria / World War II
Environmental Problems / Insect Resistance / Begin Using Again? / References
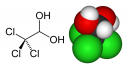
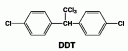
DichloroDiphenylTrichloroethane or DDT is comprised of:
- A chloral hydrate molecule [CCl3CH(OH)2] as the center “stem”
- this molecule has sleep producing properties and is known as “knockout drops” in night clubs.
- The two ends of the molecule are Monochlorobenzene (C6H5Cl)
- basically a benzene ring with a chlorine atom interchanged with a hydrogen
- The monochlorobenzene attaches to the OH group of the chloral hydrate molecule and water is released.
- It is a unique insecticide in that it kills both types of insects
- 2 types:
- 1. insects that chew plants
- these insects are killed with the stomach poison aspect of DDT when they ingest the plant.
- 2. insects that puncture plants and suck out juice
- these insects are killed as soon as they come in contact with the plant.
- 1. insects that chew plants
- 2 types:
- It also has a “residual effect” so the effects last for a long time without the need for the area to be resprayed. It can be effective 6 months or more.
- DDT is not water soluable so it needed to either be dissolved in kerosene, made into an emulsion, or the grains were chemically coated to make a wettable powder.
- Because of its insolubility it is not metabolized quickly in animals. The half life is 8 years, so it takes an animal 8 years to digest half of its total intake of the molecule.