Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen’s chemical structure is C13H18O2.
The molecule is a white crystalline powder and has a melting point between 74°C to 77°C. It is not very soluble in water but is soluble in ethanol and acetone.
The molecule is composed of a carboxylic group in the right hand corner, of a phenyl group in the middle, and of an isobutyl group to the left of the image.
Ibuprofen is made up of two separate molecules — R-ibuprofen and S-ibuprofen. These have the same structure but vary in their arrangement. In fact, they are isomers called enantiomers. The difference lies in how the atoms are connected in the second carbon from the right.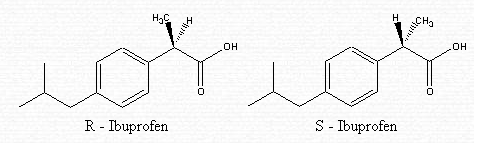
In the R-isomer, the methyl (CH3) group is in the front . In the S-isomer, the methyl (CH3) group is in the back.
