Introduction – Origins – Significant People – Chemistry
Isooctane Affects History – Advertsing – The Future – References
CHEMISTRY
There are specific chemical reactions that lead to the differences between knocking tendencies of fuels with different sizes and molecular structure. The oxidation of isooctane and n-heptane occurs when they are combined, and it creates fuel. The more isooctane the less knocking and the more heptane the more knocking there is.
The addition of one more carbon and two more hydrogens to the molecule heptane, creates isooctane. The difference in characteristics of two hydrocarbons had to do with odd versus even numbers, which is 7 versus 8, of carbon atoms in the molecules. Also, isooctane is more branched than heptane and these certain characteristics makes the difference between isooctane, which resists knocking, and heptane, which knocks readily.
Isooctane
C8H18
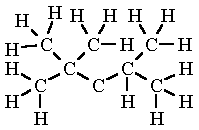
Heptane
C7H16
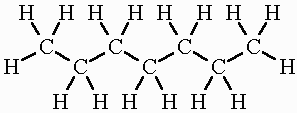
When a hydrocarbon fuel burns completely, the oxygen in the air combines with the hydrogen to form water, and with the carbon to form carbon dioxide. If the burning is not complete, then some of the carbon atoms only combine with one oxygen atom rather than two, to form carbon monoxide, Carbon monoxide is a poisonous gas emitted through the process of combustion engines.
