Introduction to THC – History of THC – THC in the News – Cancer and THC
Effects of THC – Chemistry of THC
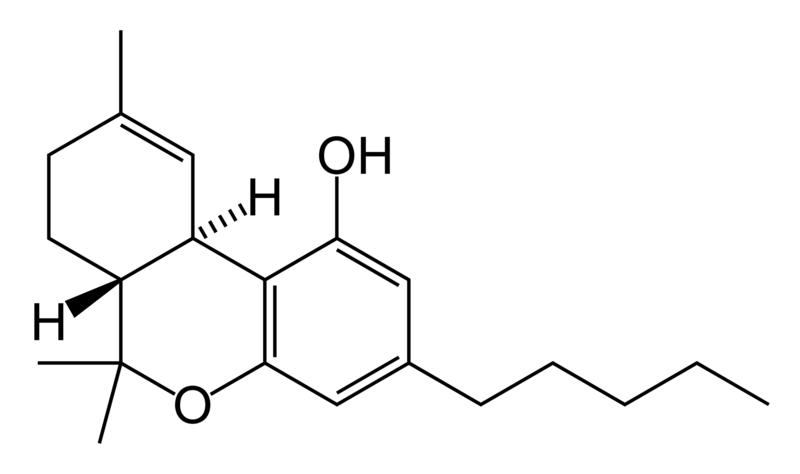
Structure of THC:
- Boiling Point 200 degrees C
- Chemical formula: C 21 H 30 O2
- The structure of THC is classified by where the double bonds occur. The molecule is classified by delta and then a number
- The molecule as two chiral carbons allowing for four stereo-isomers to form
- The double bonds in the molecule lock the atoms in place creating cis and trans isomers
- The place in which the double bond is causes one molecule of THC to be more potent then other.
- The top ring contributes to the cannabinoid activity in the receptors
- But the most important part of the molecule is the side chain, potency can be increased by adding more carbons on to it. It can be increased by 7 carbon chains
- The orientation of the side chain on the lower part of the chain plays an important role on how the receptor receives the molecule
- The configuration of the hydroxy group is critical to determining the potency
- When the receptors receive the molecule, the exact line up of the bonds and side chains determine how “high” one will get when smoking or ingesting the molecule