Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
References
- Abramson, S., Korchak, H., Ludewig, R., Edelson, H., Haines, K., Levin, R. I., et al.
(November 1, 1985). Modes of Action of Aspirin-Like Drugs. Proceedings of the National Academy of Sciences of the United States of America, 82(21), 7227-7231. Retrieved April 18, 2008, from http://www.jstor.org/stable/26342 JSTOR.
- Barry, W., Meinzinger, M. & Howse, C. (July 13, 1984). Ibuprofen overdose and exposure in utero: results from a postmarketing voluntary reporting system. The American Journal of Medicine, (77), 35-39. Retrieved April 20, 2008, from http://www.ncbi.nlm.nih.gov/pubmed/6465161 Pub Med.
- BASF Aktiengesellschaft. Technical Information: Ibuprofen. January 2004. 15 April 2008 http://www.ibuprofen-foundation.com/what-ibuprofen/documents/TIIbuprofen.pdf.
- Brunton, L. L., Lazo, J. S. & Parker, K. L. (2006). The Pharmacological Basis of Therapeutics (11th ed.). In (L. L. Brunton, Ed.). New York: McGRAW-HILL, Medical Publishing Division.
- Holubek, W., Stolbach, A., Nurok, S., Lopez, O., Wetter, A. & Nelson, L. (June 2007). A Report of Two Deaths from Massive Ibuprofen Ingestion. Journal of Medical Toxicology, 3(2), 52-53. Retrieved April 15, 2008, from http://www.ncbi.nlm.nih.gov/pubmed/18072160 Pub Med.
- Lanza, F. (July 13, 1984). Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other nonsteroidal anti-inflammatory agents. The American Journal of Medicine, (2), 19-24. Retrieved April 18, 2008, from http://www.ncbi.nlm.nih.gov/pubmed/6465160 Pub Med.
- Mar, D. (June 1981). The “Simple” Analgesics. The American Journal of Nursing, 81(6), 1206-1208. Retrieved April 18, 2008, from http://www.jstor.org/stable/3424820 JSTOR.
- Moore, N. (April 2003). Forty years of ibuprofen use. International Journal of Clinical Practice, (135), 28-31. Retrieved May 2, 2008, from http://www.ibuprofen-foundation.com/news/conferences/40yrs.pdf
- Moore, N. (2007). Ibuprofen, a journey from prescription to over-the-counter use. Journal of the Royal Society of Medicine, 100(48), 2-6. Retrieved April 27, 2008, from http://www.rsmpress.co.uk/S48-1-2.pdf
- Nelson, D. L. & Cox, M. M. (2004). Lehninger Principles of Biochemistry (4th ed.). W. H. Freeman.
- Pennisi, E. Building a Better Aspirin. Science, 280(5367), 1191-1192. Retrieved April 15, 2008, from http://www.jstor.org/stable/2896046 JSTOR.
- Rainsford, K. (April 2008). Discovery, mechanisms of action and safety of ibuprofen. International Journal of Clinical Practice, (135), 3-8. Retrieved May 2, 2008, from http://www.ncbi.nlm.nih.gov/pubmed/12723739 Pub Med.
- Smith, A. (Ed.). (2000). Oxford Dictionary of Biochemistry and Molecular Biology. New York: Oxford University Press.
- Travis, J. (2000, August 12). Ibuprofen Cuts Alzheimer Protein Build-up. Science News, 158, 7., p. 101. Retrieved April 15, 2008, from http://www.jstor.com/stable/3981212 JSTOR.
- Volans, G. & Colbridge, M. (2003). Ibuprofen Overdose. International Journal of Clinical Practice, (135), 54-60. Retrieved April 20, 2008, from http://www.joplink.net/prev/200605/ref/09-008.html
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on Side Effects
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
As with any drug, ibuprofen has some side effects. The list can be long, but some of the more common side effects are listed below.
- chest pain, weakness, shortness of breath, slurred speech, problems with vision or balance
- black or bloody stools
- swelling, rapid weight gain
- less urinating than usual
- nausea, fever, stomach pain
- sore throat, bruising, headache, chills
There have been an increasing number of ibuprofen overdose cases since the drug has become sold over-the-counter. Nevertheless, life-threatening cases of overdose have been low.
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on How is Changed History
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
There are numerous ways in which ibuprofen changed history, but some of the more notable are:
- Because the drug is nonsteroidal, and therefore does not upset the hormonal balance in the body, it can be used extensively. This allowed it to substitute other forms of drugs that were used in the past, which might have been harmful to the body.
- In certain studies, ibuprofen has shown better results than a placebo in the prevention of Alzheimer’s disease when given in small amounts over a long stretch of time.
- Ibuprofen has shown to lower the risk or even prevent Parkinson’s disease, while other drugs such as Aspirin did not have this effect.
- A research study has showed that ibuprofen works best compared to common pain killers such as generic acetaminophen and codeine for kids with broken bones, bruises, and sprains.
- Research shows that use of ibuprofen might lessen the chance of cancer and benign prostatic hyperplasia. However, it is not recommended to take ibuprofen for such purposes, but this phenomenon has rather appeared as a side effect in patients who were prescribed the medicine for other purposes.
- Most commonly,
 ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on Terms
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
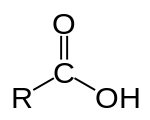 Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.
Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.

Phenyl group = functional group in which 6 carbon atoms are arranged in a cyclic ring structure.
Enantiomer = isomeric molecules with chiral properties with a mirror image relationship.
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on How it Works
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen functions in such a way that it stops the action of the enzyme cyclooxygenase. This enzyme brings about the transformation of fatty acids to prostaglandins. Therefore, ibuprofen stops the synthesis of prostaglandin, which causes its analgesic (pain-relieving) and anti-inflammatory action. More simply said, ibuprofen works by reducing the specific hormones that cause the undesired inflammation and pain in the body.
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on Chemical Structure
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen’s chemical structure is C13H18O2.
The molecule is a white crystalline powder and has a melting point between 74°C to 77°C. It is not very soluble in water but is soluble in ethanol and acetone.
The molecule is composed of a carboxylic group in the right hand corner, of a phenyl group in the middle, and of an isobutyl group to the left of the image.
Ibuprofen is made up of two separate molecules — R-ibuprofen and S-ibuprofen. These have the same structure but vary in their arrangement. In fact, they are isomers called enantiomers. The difference lies in how the atoms are connected in the second carbon from the right.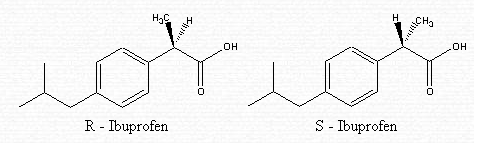
In the R-isomer, the methyl (CH3) group is in the front . In the S-isomer, the methyl (CH3) group is in the back.
[Read more →]
Categories: Ibuprofen
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References

The discovery of ibuprofen was not accidental unlike many other molecules. Its initial purpose was to treat arthritis.
It was discovered in the 1950s by Boots Group, a pharmacy chain in the United Kingdom. It was discovered by Stewart Adams and his colleague John Nicholson. Interestingly enough, Adams first tested the drug on a hangover. The drug was made available to the public almost 20 years later in 1969. During the 1970s ibuprofen would become more popular all over the world. Finally, between 1978 and 1983 the drug started to be sold over-the-counter worldwide, and in 1984 the United States recognized it as an over-the-counter drug as well.
[Read more →]
Categories: Ibuprofen
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen is a drug that contains non-steroidal anti-inflammatory properties as well as analgesic (pain relieving) and fever reducing properties. It is most commonly sold under the trademarks Advil and Motrin. Most commonly, ibuprofen is seen as an over-the-counter drug, but it may also be sold under a prescription if larger doses are necessary.
It is used to temporarily relive minor pains such as:
- Headaches

- Muscular aches
- Minor pain or arthritis
- Toothache
- Backache
- Colds
- Menstrual cramps
- Temporarily reduces fever
The chemical name of ibuprofen is 2-(4-isobutylphenyl) propanoic acid.
Over-the-counter doses of ibuprofen are considered to be low, and these usually range between 200 mg to 400 mg. The duration of such a dose lasts on average between 4 to 8 hours.
[Read more →]
Categories: Ibuprofen

 ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking. Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.
Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.



