Entries from April 2008
April 24th, 2008 · Comments Off on Electricity & Power
|| INTRODUCTION || DISCOVERY || STRUCTURE || “SMART” CLOTHES ||
|| ELECTRICITY & POWER || CANCER & DISEASE TREATMENT || REFERENCES ||
—————————————————————————————————————–
Electric Vehicles
The current fossil fuel crisis is producing the need for more efficient and alternative energy solutions. Carbon nanotubes and their application to electrical devices may prove to be the solution scientists have so eagerly sought.
The separation between carbon nanotubes in a nanotube capacitor is only a nanometer whereas the separation in a traditional modern capacitor is a micrometer. The extremely small distance increases the electrical capacity.
- A large charge can be put into a nanotube capacitor with only a few administered volts. Supercapacitors such as these could be used in lieu of modern batteries: they have a higher power capability and more capacity.
- These possibilities lead to usable, electric only, cars. The extraordinary power of the battery would allow a car to accelerate rapidly and be able to travel over relatively long distances as opposed to current battery powered cars which can only operate at certain speeds for relatively short amounts of time. The batteries operate at low voltage levels and work in temperatures up to 350 degrees Celsius.
Electronic devices decrease in size by half every three years!
- Carbon nanotubes will allow scientists to keep with this trend – all silicon based electronics could be phased out within a decade as nanotube circuits continue to be refined.
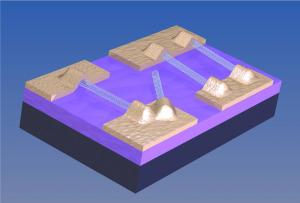
LCD (liquid crystal display) televisions may be eclipsed by TVs that use carbon nanotubes as an outer layer on their display surface.
- Aligned nanotube displays have –
- Much larger operating temperature range
- All these characteristics originate from nanotubes’ unique surface electric and electromagnetic properties
The Light Bulbs of the Future
Nanotube coated light bulbs –
- Will be made available for commercial use in the near future
- In laboratory tests had lifespan of greater than 8000 hours
- They have the long lifespan without the environmental impacts of mercury-fluorescent light bulbs.
- Have the output to illuminate stadiums
[Read more →]
Categories: Carbon Nanotubes
April 24th, 2008 · Comments Off on Cancer & Disease Treatment
|| INTRODUCTION || DISCOVERY || STRUCTURE || “SMART” CLOTHES ||
|| ELECTRICITY & POWER || CANCER & DISEASE TREATMENT || REFERENCES ||
—————————————————————————————————————–
Perhaps the most important use of carbon nanotubes is selective cancer and disease treatment.
- Unique properties of nanotubes make them ideal carriers of treatment into cancerous cells.
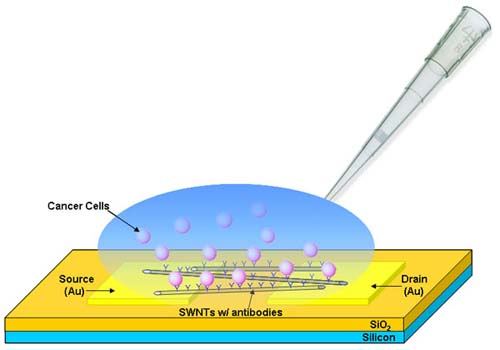
- By attaching antibodies to the nanotubes cancerous cells can be targeted.
- Because nanotubes are especially receptive to near-infrared light, they make targeting cancerous cells extremely effective and minimize damage to surrounding cells.
[Read more →]
Categories: Carbon Nanotubes
History of Perfumes/ Perfume Ingredients/ The Family of Esters/ Formula of Benzyl Acetate/ Properties of Benzyl Acetate/ Uses of Benzyl Acetate/ Benzyl Acetate in Perfumes
“The earliest recognizable chemists were women, the perfume-makers of Babylon, who used the earliest known stills to produce their wares. The first individual chemist known to history was “Tapputi, the perfume-maker”, who was mentioned on a cuneiform tablet from the second millennium BCE in Mesopotamia”
~ Paul Strathern, Mendeleev’s Dream
Ancient people realized that there is a pleasure in the aroma of a flower.
People in the area of Indus, Nile and Tigris used herbs and spices for rituals.
The Classical world of Greece and Rome built knowledge of spices, herbs, oils, and developed the process of distillation.
Since perfumes were still a very expensive product, until the 19th century they were used mostly by the wealthiest groups of society.
After WWII the fragrance industry developed greatly with the production of synthetic aromas. These new synthesis increased the variety of scents available and decreased the cost of production, since obtaining odorants from natural sources involves very expensive process.
After 1960 the economies of US, Western Europe, and Japan experienced upward development. Accumulation of disposable income stimulated customer purchases. Now more people could afford luxuries like perfumes. Consequently, perfumes became a personal care product and a common present for family holidays.
Geographical distribution of current sales: Developed world – 80%
32% – USA
26% – Asia Pacific
25% – Western EU
17% – Rest of the World
[Read more →]
Categories: Perfume (Esters)
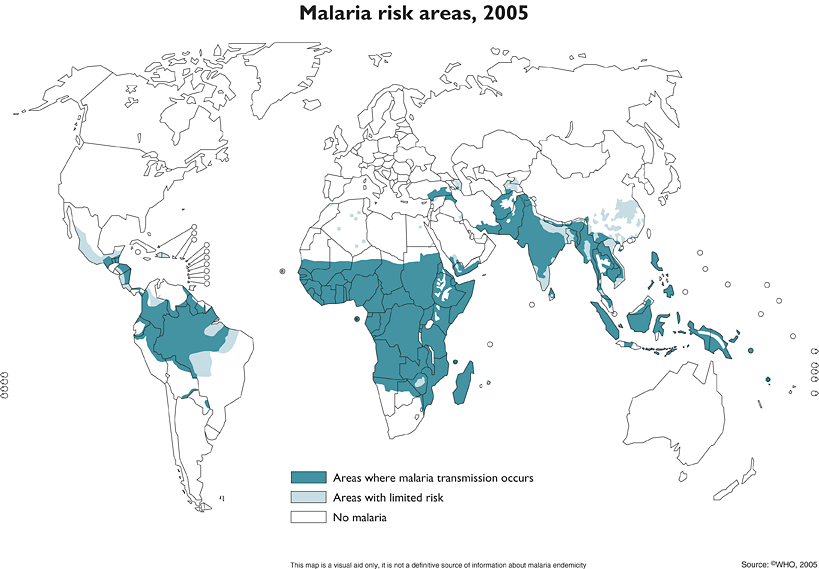
April 23rd, 2008 · Comments Off on Malaria
Introduction / DDT molecule / Malaria / World War II
Environmental Problems / Insect Resistance / Begin Using Again? / References
- Over 1 million people die from Malaria each year
- Much of Europe and the America’s were malarial until the early 1950s
- Most people associate the disease with developing and tropical countries but it was only recently been eradicated
- Two ways to fight the disease:
- 1. Use drugs such as quinine to treat already infected patients
- 2. Try to destroy the carriers of the disease: anopheles mosquito

- In 1944, DDT was proposed as a way to eradicate Malaria after the success of killing mosquitoes and protecting soldiers during WWII
- It was first used again in Italy. Spraying began in 1945 and by 1948 and after spraying half a million pounds over 135,000 miles, the malarial death in Italy was zero.
- After this success the World Health Organization (WHO) began to implement malaria control programmes in several countries.
- Example of the successes of DDT in Malaria eradication:
- South America shortly after the first spray in 1946, the number of Malaria cases dropped 1/3 from what it was in 1942.
- Sri Lanka began spraying in 1946 as well. In 10 years the number of cases of Malaria was cut from 3 million to 7300 and had eliminated ALL Malaria related deaths. And by 1964 the number of cases was just 29.
- In India it was estimated that around 75 million contracted the disease each year and about 800,000 people died. Through the use of DDT the number of contracted malaria cases dropped from 75 million in 1951 to 50,000 in 1961.
- Malaria was successfully eradicated from 10 countries (4 in Europe and 6 in Americas and Carribean)
- In 1951:
- it was discovered that the anophelines mosquitoes in Greece came back to spraying sites within days
- In 1953:
- it was clinically proven that the anepheles mosquito was physiologically resistant to DDT
- In 1956 the Malaria rates began to rise again because of:
- The successes many countries stopped the intensive spraying because they thought that they had effectively stopped the spread of the disease.
- And because of the developing resistance to the pesticide.
- The World Health Organization decided to blast these countries with DDT where Malaria was increasing to hopefully kill the mosquitoes before they had the chance to develop the resistance. But many third world countries where this treatment was needed didn’t have the capabilities for such a blast.
- Slowly the Eradication campaign turned into control and in sub-saharan Africa it was reduced to a Containment Campaign.
- Then in 1962 Rachel Carson published Silent Spring and the environmental battle on DDT began and it was subsequently banned by the U.S. in 1972.
[Read more →]
Categories: DDT
Introduction | Discovery | Chemical Composition
Usage | Historical Significance
Heroin is an opiate made from the chemical morphine, extracted from the opium poppy. The drug is extremely fast acting and within seconds results in the feeling of extreme well-being and euphoria.
Heroin is very addictive, both physically and psychologically, and its effects diminish with increased tolerance. Therefore, users must consistently increase the dosage of heroin in order to achieve the same levels of well-being.
In the United States, Heroin is considered a schedule I drug which makes its possession and distribution illegal without a DEA license. Under US law, the possession of heroin results in imprisonment for a minimum of 5 years
.
[Read more →]
Categories: Heroin
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
Ozone is a very potent oxidizing agent, which can turn an olefin into a ketone, a carboxylic acid or an aldehyde. It is also used as a commercial bleaching agent, effective on organic compounds. Ozone is also put in drinking water so it can act as a germicide and sterilize the water. While in the water it can also take away unfavorable tastes and smells. Ozone is used in whiskey distilleries as a way to destroy the empyreumatic taste of whiskey. It is useful as a decomposition agent. It can decompose dangerous organic and inorganic chemical wastes such as phenols, pesticides, detergents, and cyanides. Ozone can also help precipitate out unwanted heavy metal ions and hydroxides, like iron and manganese. With ozone, one can control not only the formation of slime, but also the odor in sewage treatment and in manufacturing industries.
[Read more →]
Categories: Ozone
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
In the late 1920s, there was a need for a safe refrigerant. Thomas Midgley, who was working for General Motors at the time, found the answer in chlorofluorocarbons, or CFCs. This seemed to be the perfect response because CFCs are inert in the troposphere. So they were used in air conditioning, aerosols and other appliances. However, in 1974 Mario Molina and Sherry Rowland put forth the hypothesis that the decline in concentration of the ozone layer was due to the CFCs. This process of man-made destruction of ozone happens because CFCs are long lasting, inert molecules that can accumulate. Winds carry the CFCs up to the stratosphere, where a UV ray breaks off a chlorine from the CFC, which is highly reactive and takes one of the oxygen atoms in ozone and bonds to that. Then the oxygen atom bond to another oxygen atom and the chlorine is free to repeat the process. A single atom of chlorine can kill tens of thousands of ozone molecules before it is removed. The CFCs travel to Antarctica because of wind patterns and accumulate there, causing the ozone hole. The hole is also due to chlorine in reservoir species, ClONO2 and HCl, where they get into polar stratospheric clouds and can go reactions which release the chlorine atom. Because natural ozone formation and destruction needs the Sun’s rays, the destruction of the ozone layer can have an accumulation effect if the levels are never recovered between winters. In the decade following Molina and Rowland’s findings, industry battled science about the production of CFCs. In the early 1980s evidence proving their point mounted. With data going back to 1957, there was a clear decline in ozone concentration and really picked up in the early 1980s. Also, findings showed that in places with decreased reservoir species had an abundance of active chlorine compounds and a decreased ozone level. So, in 1987 the Montreal Protocol was held and they decided to cut CFC production in half. This was a monumental occasion because it was the first international attempt to prevent ozone destruction. Ozone destroying chemicals dropped ninety-five percent since the late 1980s, and CFCs were eventually banned in the United States.
History also effects ozone in the troposphere. With the mass production of cars in the early twentieth century came pollution. The combustion of fossil fuels in automobiles creates nitrogen dioxide, which breaks apart because of UV light into a nitrogen oxide radical and oxygen radical. The oxygen radical then bonds with an oxygen molecule, creating ozone.

�
[Read more →]
Categories: Ozone
April 23rd, 2008 · 1 Comment
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
The Ozone layer is located around twelve to twenty five kilometers, or eight to fifteen miles, above the Earth’s surface. The layer protects us from the harmful UV rays by absorption. If the UV rays would get to the Earth’s surface, then life as we know it today would not exist. Also, if the UV rays can get to the surface now, it will have a severely negative impact on DNA and RNA. One billion tonnes of ozone is naturally formed and destroyed a year. Ozone is formed naturally when a UV ray breaks the bond of oxygen-to-oxygen, leaving two atoms. Then, with the help of another atom that is bonded to another oxygen molecule, the oxygen atom forms a bond to the oxygen molecule. It is naturally destroyed when a UV ray breaks one of the bonds in ozone, leaving an oxygen molecule and an oxygen atom. Each of these processes happen at the same rate, leaving a constant amount of ozone in the stratosphere.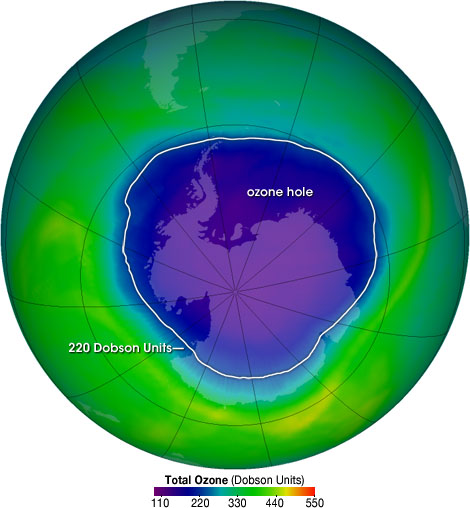 �
�
[Read more →]
Categories: Ozone
April 23rd, 2008 · Comments Off on Ozone in the Troposphere
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
Lower troposphere ozone impacts human health, crops, forests and natural ecosystems, so there is effort to keep the ozone levels at a minimum. Both industrialized and developing countries have been found to have potentially harmful levels of ozone. Since the 1970’s there has been a constant increase in ozone concentration, raising concerns. Ozone irritates eyes and nasal passages, and people with asthma or heart disease are especially susceptible to the negative effects. If one is exposed to ozone levels at .3 ppm for one to two hours, fatigue and respiratory difficulties result. Ozone is also very poisonous to plants, especially leafy ones like tobacco and tomatoes. It reduces photosynthesis, which causes leaves to turn yellow.

�
[Read more →]
Categories: Ozone
April 23rd, 2008 · Comments Off on Ozone History
Ozone History | Ozone in the Troposphere | Ozone in the Stratosphere | History Effects Ozone | Ozone’s Other Uses
After a lightening strike, there is a peculiar odor that man has been aware of for centuries. It has been mentioned works such as The Illiad and The Odyssey. In 1785 Martin van Marum studied the passing of an electrical spark through diatomic oxygen and noticed a sweet smell. He attributed the smell to the electricity, calling it the “electrical odor.” It was not until Christian Schöbein studied the odor that he realized it did not come from the electricity, but was a different molecule. He named it ozone after the Greek word ozein, which means to smell. Schöbein is also the founder of guncotton, when he wiped nitric acid with a cotton cloth in his kitchen. The actual structure was not determined until T. Sterry Hunt hypothesized it was three oxygen atoms. The fact that ozone is so widespread was not realized until 1850. Gordon Dobson made the first tool to measure ozone and surface measurements began in the 1860s. The discovery of the ozone layer in the stratosphere did not come until the early Twentieth century.

�
[Read more →]
Categories: Ozone








 �
�
