Entries from April 2008
April 22nd, 2008 · 1 Comment
Introduction to Coal | Coal as a Fuel|Coal Affects History| Coal Mining and Effects| Environmental Effects| History Affects Coal|References|
Coal Mining
-
Contamination of Ground water systems: When coal surfaces are exposed to the elements the FeS2 (also known as pyrite and fools goal) on the coal surface then reacts with water to create sulfuric acid. Once the water drains from the mine the acid enters waterways and groundwater systems. This is not a onetime event either. Sulfuric acid will be produced every time rain falls on the exposed mine.
-
Destruction of above ground structures: Collapses of mines have caused major damage to infrastructure in the surrounding areas. Occasionally, houses and other buildings can sink into the ground mandating the relocation of anywhere from one or two families to a small town. Additionally, mine collapses of significant size have on occasion caused small earthquakes. In 2008, Germany suffered an earthquake that registered 4.0 on the Richter scale as the result of a suspected mine collapse.

Combustion of Coal
The harmful impacts of burning coal are a result of the gasses that are produced as a result of the combustion reaction. These products are principally oxides of carbon, nitrogen, and sulfur some of which are greenhouse gasses. However, additional products also include hydrides and nitrides of sulfur and oxygen. Examples include toxic gasses such as hydrogen cyanide.
-
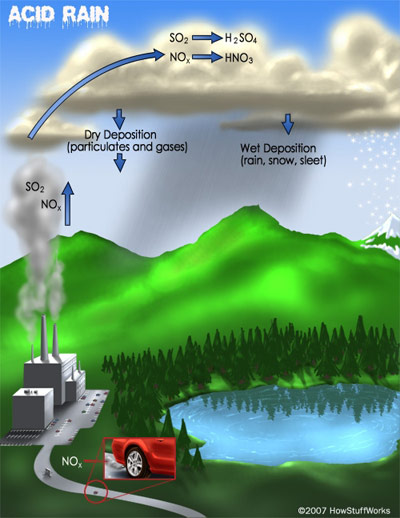
Acid Rain: Acid rain may be produced from SO2 synthesized as a result of burning coal. The SO2 reacts with oxygen in the atmosphere to form SO3 (2SO2 + O2 → 2SO3). The SO3 in turn reacts with the water in the atmosphere to produce sulfuric acid (SO3 + H2O → H2SO4), which falls to the earth as acid rain.
-
Other hazardous waste products: the waste products of coal plants include many heavy metals (examples: As, Pb, Hg, Cr, Cu) as well as several naturally occurring radioactive such as uranium. If not properly disposed these byproducts pose severe risks to people and the environment.
[Read more →]
Categories: Coal
April 22nd, 2008 · Comments Off on Coal as a Fuel
Introduction to Coal | Coal as a Fuel|Coal Affects History| Coal Mining and Effects| Environmental Effects| History Affects Coal|References|
Early Uses
Archaeologists speculate that coal was first used as fuel as early as 120,000 years ago when it was used for campfire cooking in Germany. Other early uses of coal for fuel have been evidenced in China as early as 10,000 B.C. and in Britannia prior to the Roman invasion where it was used as a source of heat, the Aztecs also used coal for that purpose. Early uses of coal were simplistic, since coal is a ready-combustible material lighting a fire was enough to unlock coal’s potential as a source of heat and a cooking implement.
Industrial Revolution

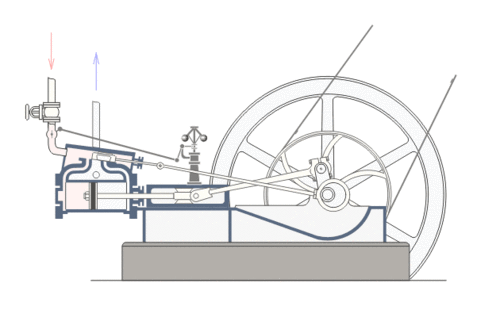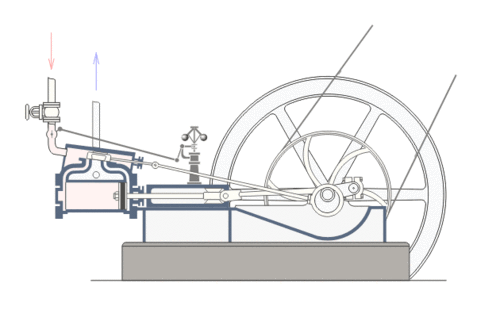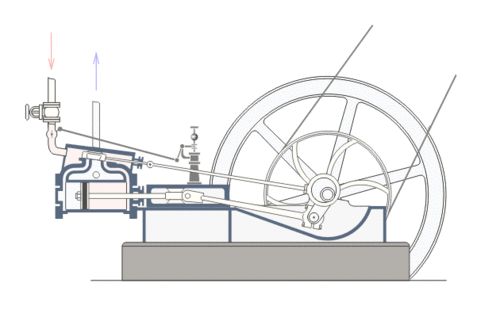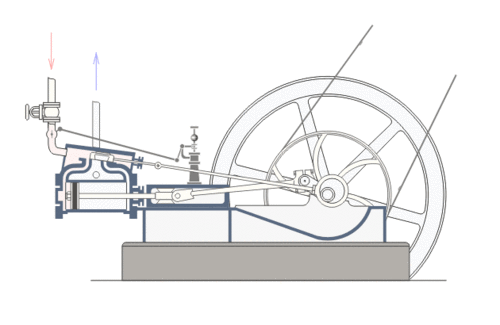
Coal was the primary fuel used to power the steam engines of the Industrial Revolution (such as the Watt Steam Engine pictured above). The steam engine implemented a thermodynamic process where coal (usually bituminous or sub-bituminous) is used to heat water in a boiler (or “steam generator”) which in turn produces steam. The steam places pressure on a turbine blade or piston. The motion of the blade or piston can then be used to move gears and wheels and thus, power machinery.
Present Day
In the contemporary industrial world, coal is still a popular source of fuel due to its abundance and low cost. Coal plants provide most of the electricity in the United States and many other countries. The basic principle of the steam engine is still used. The principle difference is that the turbines are used to power electrical generators rather than gears and wheels. Additionally, coal is now industrially pulverized before being burned. Most coal plants in the United States are in continuous use and require daily shipments of 10,000 tons of coal per day (or more) to continue to operate.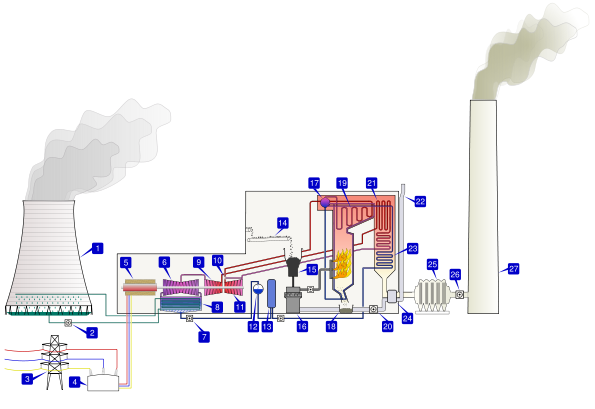
Other Contemporary Examples
- Gasification: Cheaper alternative to natural gas and oil. Cleaner then burning coal the conventional way.
- Liquefaction (Coal-to-Liquids or CTL): Conversion of coal to liquid fuels similar to gasoline. Environmentally unsound but a potential means of stabilizing oil prices in the future.
- Clean Coal Technology: See History Affects Coal
[Read more →]
Categories: Coal
Introduction to Coal | Coal as a Fuel|Coal Affects History| Coal Mining and Effects| Environmental Effects| History Affects Coal|References|
Coal Mining
Concerns over the safety of miners have led to a number of safer mining techniques where the work is done primarily by machine.
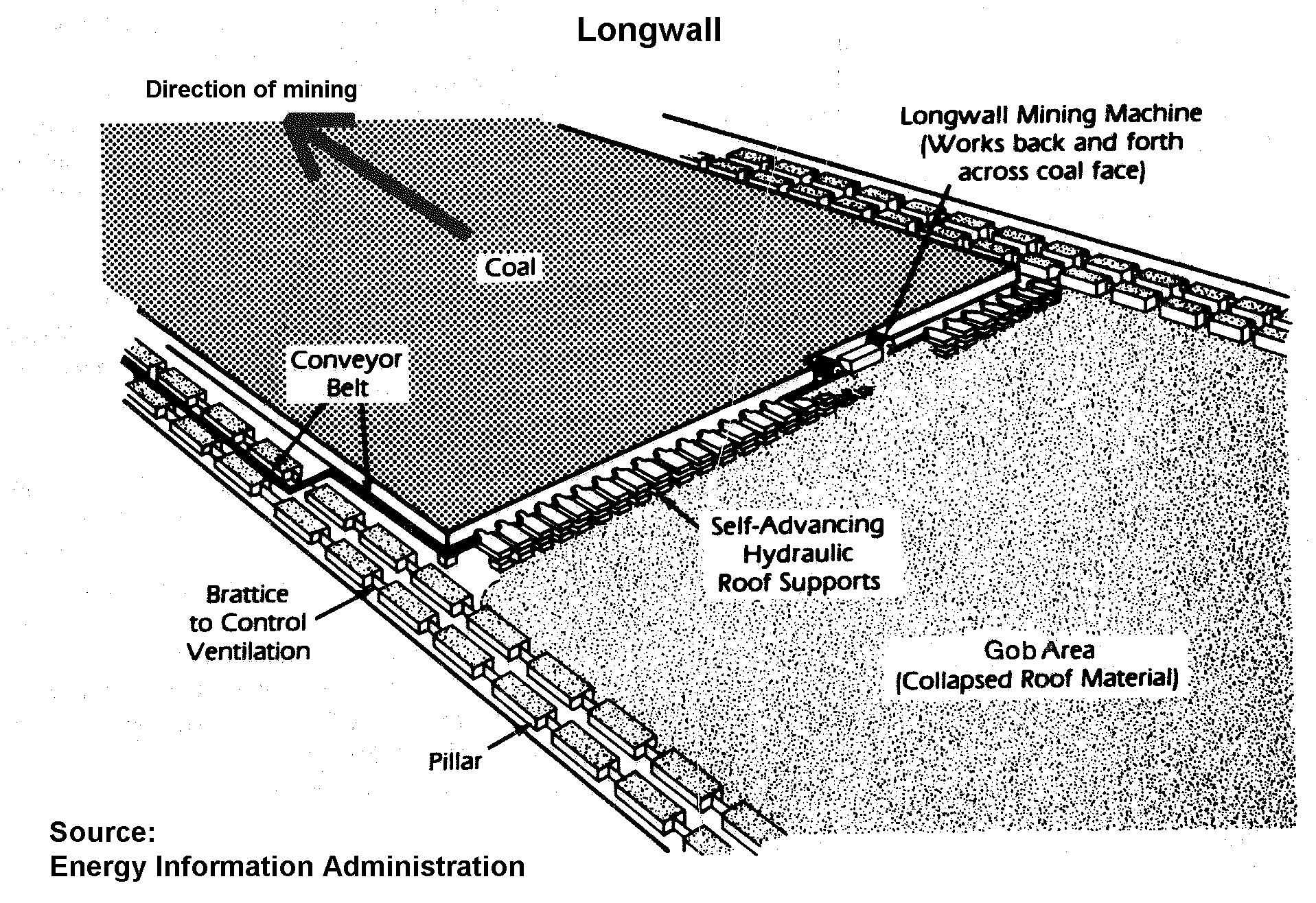
Environment
The Surface Mining Control and Reclamation Act of 1977: This piece of U.S. Federal legislation set mandatory provisions for the monitoring of active coal mines and reclamation of abandoned and/or collapsed coal mines in order to mitigate harmful environmental affects. It has been used a model for similar legislation in several other countries.
Clean Coal Technology
Clean coal technology was developed as a means of making coal pants more environmentally friendly and is becoming common practice. Clean coal technology aims to both recover the CO2 released during the coal combustion reaction to reduce the carbon cost and to remove sulfur dioxide from the reaction by cleansing the coal of minerals and impurities. The process is promising but has been criticized by some environmentalists who claim: “There is no such thing as clean coal.”
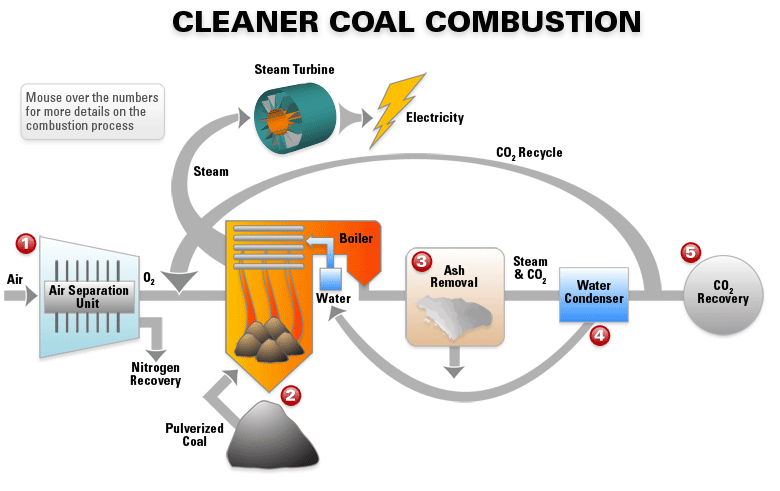
Click on the diagram for a clearer view!
[Read more →]
Categories: Coal
Introduction to Coal | Coal as a Fuel|Coal Affects History| Coal Mining and Effects| Environmental Effects| History Affects Coal|References|
Structure
Coal is a fossil fuel formed from plant remains that were trapped in mud and therefore not oxidized. It is technically a sedimentary rock with a chemical structure similar to that of a polymer. Its structure varies based on the age of the coal and therefore the amount of pressure applied to it over time. The main types of coal are listed below from youngest:
· Peat (technically a precursor to coal)
· Lignite
· Sub-bituminous coal
· Bituminous coal
· Anthracite (pictured below)
· Graphite

The structure of coal varies by type and even sometimes within a given age group. However, the typical coal structure consists of numerous aromatic rings of five or six carbons bonded with principally hydrogen, nitrogen, sulfur, and oxygen atoms. Nitrogen, hydrogen, and sulfur are responsible for the majority of coal’s chemical properties.

History
The nitrogen and oxygen atoms in coal result in a readily combustible structure which has made coal a popular fuel and source of heat throughout history. Coal was first mined as fuel as far back as 10,000 years ago in China and today is the primary fuel implemented in the production of electricity worldwide. During the last three hundred years, coal played an important role in the technological advances, culture, and the global economy. In the present day coal is a center of controversy; coal mining is notorious for the risks involved and the associated social woes. Additionally, burning coal produces greenhouse gases and is therefore, a contributing factor to global warming. However, political activism and chemical research have done much to alleviate these concerns and coal continues to be an important and improved source of fuel in the twenty first century.

[Read more →]
Categories: Coal
Introduction to Coal | Coal as a Fuel|Coal Affects History| Coal Mining and Effects| Environmental Effects| History Affects Coal|References|
Industrial Revolution
As previously mentioned, coal played a pivotal role in the industrial revolution as fuel for the steam engine that was driving the movement. However, beyond its role as a fuel coal also had a tremendous impact on the global economy, politics, and foreign relations during the era of the industrial revolution.
Britain’s abundant supply of coal is the reason that it became the first industrial power. After the invention of the steam engine Britain’s abundant supply of coal allowed it the resources to set up factories and begin the process of industrialization. Many other nations, such as the United States, also benefited from their vast coal resources and were quick to industrialize. However, for other nations shortages in coal resources proved a significant obstacle to industrialization of infrastructure and modernization of their economy. This impacted the balance of power among the imperialist nations of the world. Nations that were able to industrialize were able to produce goods and, perhaps more importantly, munitions faster than their peers. This gave Britain, the United States, and other nations a decided military advantage against unindustrialized opponents. Improvements in the production and quality of arms as a result of the steam engine and the industrial revolution help account for the expansion and maintenance of the British Empire as well as the high casualty rates in the U.S. Civil War.
However, the eagerness of unindustrialized countries to catch up with Britian opened up a lucrative market in trading coal. This market still exists today as many countries import coal as a (as previously mentioned) inexpensive fuel source for power plants. In contemporary times the two largest producers of coal are the United States and Australia. The two exported a combined 308 million tons of coal in 2005.
Transportation Innovations
In the early 19th century coal fueled the newly invented steam boat and steam locomotive. The steam locomotive in particular revolutionized transportation. Both the steam engine and boat were powered by a steam engine similar to the one powering Britain’s factories. The steam engine allowed for faster and cheaper transportation of goods from one point to another. It was both faster and stronger (could lug more carts) then previous models of train engines. Ironically, the first material to benefit from the new mode of transportation was coal. Steam locomotives transported coal from the mines to rivers (and later canals) where the valuable commodity was shipped to factories.

In the present day, coal is still being used to improve transportation. As previously mentioned, scientists have successfully created a liquid fuel via liquefaction that can be used to power automobiles. It is being investigated as a cheap alternative to less abundant petroleum. Unfortunately, an affordable process of making the liquid fuel environmentally friendly has not yet been discovered.
Culture
In the United States Santa Claus stuffs coal in the stockings of naughty children. However, in Scotland coal is a traditional New Year’s gift and represents warmth in the year to come.

[Read more →]
Categories: Coal
Introduction | What is Caffeine: Molecule Structure | Stimulating Science: The Properties of Caffeine | Discovery: The Magical Bean | Coffee Creates a Social Lifestyle in Europe |Colonization and Coffee|Coffee’s Impact on the Nation of Brazil|Coffee Industry Today| Conclusions
There is no doubt that human beings have consistently consumed caffeine since the Stone Age. It is hypothesized that ancient people discovered that chewing on certain plants had stimulating effects on the body and mind. Myths of the initial discovery of the coffee bean and its properties vary from culture to culture. Coffee is indigenous to Ethiopia, therefore it comes as no surprise that one well known legend comes from this nation. The Ethiopian myth describes the story of a goatherd named Kaldi, whose goats nibbled on a bush covered in red berries. After the goats had consumed the berries, they became frisky and started to dance on their hind legs. Kaldi was intrigued by the strange actions of his goats and decided to try the berries for himself. After tasting the magical berries he too became energized and started to dance with his goats. A local monk noticed the goatherd’s behavior, and came to the realization that the invigorating effects of this bean could help monks remain alert and awake during long religious sessions of prayer. It was then that the monk brewed the first cup of coffee.

The consumption of coffee spread across northern Africa, and then into Arabia. And by the sixteenth century, coffee had become a staple throughout the Arab world. So much so that in countries such as Turkey the drink was considered to be as important as bread or water. The popularity of coffee can not only be contributed to the beneficial effects of the caffeine, but also to the religious ties in the Middle East to Islam. Islamic law prohibits the consumption of alcohol; therefore coffee provided a substitute. As the popularity of the beverage increased, the western world began to take notice.
[Read more →]
Categories: Caffeine
Introduction | What is Caffeine: Molecule Structure | Stimulating Science: The Properties of Caffeine | Discovery: The Magical Bean | Coffee Creates a Social Lifestyle in Europe |Colonization and Coffee|Coffee’s Impact on the Nation of Brazil|Coffee Industry Today| Conclusions
Caffeine is a psychoactive drug that is believed to be one of the most commonly used drugs around the world. After one ingests caffeine, it enters the bloodstream within ten minutes, and remains in one’s body for around twelve hours. “Caffeine has powerful psychological effects on humans and animals. It stimulates heart muscles and relaxes certain structures that contain smooth muscle, including the coronary arteries and bronchi. It is also a diuretic” (Smith, 2004, p 153).
It is also a central nervous system stimulant. Caffeine blocks the effect of the molecule adenosine in the brain, as well as in other areas of the body. Adenosine, as described in Napoleon’s Button’s is “a neuromodulator, a molecule that decreases the rate of spontaneous nerve firing and thus slows the release of other neurotransmitters; therefore can induce sleep” (Le Couteur & Burreson, 2003, p 261). Caffeine interferes with this process by binding to the adenosine receptors in the nerves, and therefore, the effects of adenosine that render one tired or sleepy cannot take place. Adenosine receptors are not only located in the brain, and can be found in other parts of the body. When caffeine molecules occupy these receptors, one will experience a “caffeine buzz” – which consists of increased heart rate, and the constriction or opening of blood vessels. (Le Couteur, 2003, p 261). One can observe below the similarities of the structures of adenosine and caffeine.
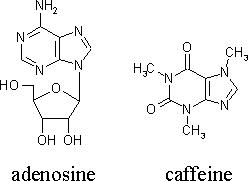
[Read more →]
Categories: Caffeine
Introduction | What is Caffeine: Molecule Structure | Stimulating Science: The Properties of Caffeine | Discovery: The Magical Bean | Coffee Creates a Social Lifestyle in Europe |Colonization and Coffee|Coffee’s Impact on the Nation of Brazil|Coffee Industry Today| Conclusions
Caffeine is a naturally occurring molecule that can be found in over sixty different plants. The caffeine acts as a natural pesticide, and protects the plants by paralyzing or killing insects that attempt to feed on the plant. The most widely used caffeine containing plants are coffee, tea. The caffeine molecule is classified as an alkaloid, meaning that it is a nitrogen-based compound that is extracted from plants. The chemical formula for caffeine is C8H10N4O2, is found in coffee.
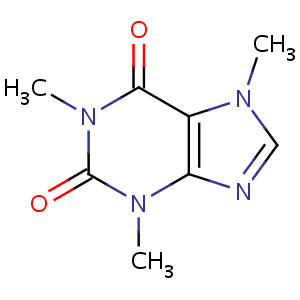
Caffeine, in its pure form, is found as odorless bitter white crystals. Pure caffeine is very soluble in hot water, which is why drinks like coffee and tea contain high amounts of caffeine. The melting point is 238° C (460° F). On average, a cup of coffee contains around 100 mg of caffeine. In contrast, the average cup of tea contains around 40 mg and the same amount can be found in a 12-ounce cola beverage.
caffeine3.txt
[Read more →]
Categories: Caffeine
Introduction | What is Caffeine: Molecule Structure | Stimulating Science: The Properties of Caffeine | Discovery: The Magical Bean | Coffee Creates a Social Lifestyle in Europe |Colonization and Coffee|Coffee’s Impact on the Nation of Brazil|Coffee Industry Today| Conclusions

Caffeine is one of the most widely consumed molecules in the world, and has been for centuries. It has remained popular because of its many positive effects on the body, which include stimulating the central nervous system, and making one more alert and giving one a boost of energy. About 90% of Americans consume caffeine every single day, and it has been estimated that over 120,000 tons of caffeine is consumed around the global per year. This makes caffeine America’s most popular drug by far. Caffeine is most commonly consumed in the form of coffee, tea, or chocolate. Although caffeine in the form of tea or chocolate have both impacted the history of the world significantly, this paper will focus on the major effects coffee has had on society and how the beverage has shaped many aspects of our collective history. Coffee has impacted the world socially, economically and politically over the centuries, influencing trading, colonization, and the development of countries as well as new industries.
[Read more →]
Categories: Caffeine
April 22nd, 2008 · Comments Off on DDT molecule
Introduction / DDT molecule / Malaria / World War II
Environmental Problems / Insect Resistance / Begin Using Again? / References
DichloroDiphenylTrichloroethane or DDT is comprised of:
- A chloral hydrate molecule [CCl3CH(OH)2] as the center “stem”
- this molecule has sleep producing properties and is known as “knockout drops” in night clubs.
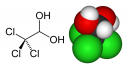
- The two ends of the molecule are Monochlorobenzene (C6H5Cl)
- basically a benzene ring with a chlorine atom interchanged with a hydrogen
- The monochlorobenzene attaches to the OH group of the chloral hydrate molecule and water is released.
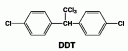
- It is a unique insecticide in that it kills both types of insects
- 2 types:
- 1. insects that chew plants
- these insects are killed with the stomach poison aspect of DDT when they ingest the plant.
- 2. insects that puncture plants and suck out juice
- these insects are killed as soon as they come in contact with the plant.
- It also has a “residual effect” so the effects last for a long time without the need for the area to be resprayed. It can be effective 6 months or more.
- DDT is not water soluable so it needed to either be dissolved in kerosene, made into an emulsion, or the grains were chemically coated to make a wettable powder.
- Because of its insolubility it is not metabolized quickly in animals. The half life is 8 years, so it takes an animal 8 years to digest half of its total intake of the molecule.
[Read more →]
Categories: DDT



















