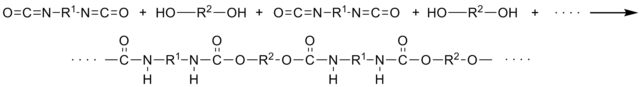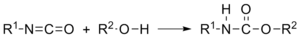May 6th, 2008 · Comments Off on Introduction ·
Introduction | Keratin | Why Wool? | A Sheep Market | Wool War I |References
Keratin and the Role of Wool in Medieval Europe
When one thinks of triangular trade, the concept that comes most readily to mind is the African slave trade. The African slave trade consisted of trade between manufacturing countries of Northern Europe, the slave traders of the West African coast and the European colonies in the Americas. This triangular system of trade began in Europe where manufactured goods were produced for sale in West Africa. Slaves were then transported from Africa to the colonies in America, where they labored on plantations, producing raw materials needed for manufacturing products. These raw materials were then sent to Europe as the last leg of the journey. This triangular trade, however, was not the first that stimulated the English economies of Northern Europe. Centuries earlier a triangular trade involving English wool drove the English economy and led her to war. Set up by the Norman invasion of England in 1066, the wool trade drove the economies of England, France and parts of the Low Countries, developing a symbiotic relationship between the economies of the three regions. When France attempted to interfere with the balance of power, England responded with the declaration of war that would last 116 years, the conflict known as the Hundred Years War.
Categories: Keratin · Uncategorized
May 6th, 2008 · Comments Off on References ·
Introduction to Coal | Coal as a Fuel|Coal Affects History| Coal Mining and Effects| Environmental Effects| History Affects Coal|References|
-
Church, R. (1986). The history of the British coal industry, vol. 3. 1830-1913. Oxford, UK: Clarendon Press.
- Cross, P.S. (1993). Clean-coal technology: State regulators respond. Public Utilities Fortnightly, 131, 42-45.
- Douglas, J.H. (1973, July 7). Coal: The stopgap fuel: Maybe. Science News, 104, 10-12.
- Kurtenbach, E. (2007, October 28). Despite worries of climate change, the world’s addiction to coal is increasing. The Salt Lake Tribune.
- Lagowski, J.J. (Ed.). (2004). Chemistry foundations and applications, vol. 1. A-C. New York, NY: Macmillan Reference USA
- Langton, J. (2000). Proletarianization in the industrial revolution: Regionalism and kinship in the labor markets of the British coal industry from the seventeenth to the nineteenth centuries. Transactions of the Institute of British Geographers, 25, 31-49.
- Lienhard, J.H. (1999). “I sell here, sir, what all the world desires to have: Power.” Retrieved April 28, 2008, from University of Houston, Engines of Our Ingenuity web site: http://www.uh.edu/engines/powersir.htm
- McGuire, P.A. (2001, May 28). Coal gets cleaner: And better connected: Fresh technology and a friend in the white house give it new respectability. Business Week, 82.
- Mead, J.S. (2001, March 19). The coal is there for us: Will the technology be? St. Louis Post- Dispatch, pp. E7.
- Moore, C.A. (2000). New showdown over coal. International Wildlife, 30, 26-31.
-
Peckman, J. (2002, July 22). Ultra-clean fuels from coal liquefaction: China about to launch big projects. Diesel Fuel News.
- Peterson, I. (1983, September 17). The dirty face of coal. Science News, 124, 189.
- Simons, H. (1955, February 5). Coal is mineral storehouse. The Science News-Letter, 67, 90-91.
- Thomsen, D.E. (1986, November 8). Rolling with coal. Science News, 130, 298-300.
- Torrens, I.M. (1990). Developing clean coal technologies. Environment, 32, 10-33.
- Turnbull, G. (1987). Canals, coal and regional growth during the industrial revolution. The Economic History Review, 40, 537-560.
- Valenti, M. (1992). Coal gasification: An alternative energy source is coming of age. Mechanical Engineering, 114, 39-44.
- International energy annual 2005: World carbon dioxide emissions from the use of fossil fuels. Retrieved April 29, 2008, from Energy Information Administration: Official Energy Statistics from the U.S. Government, World Carbon Dioxide Emissions: http://www.eia.doe.gov/iea/carbon.html
(Note: There were no page numbers given for two of the newspaper articles cited. I apologize for any inconvenience. If you have any questions please e-mail me: pkiffer@gmail.com)
Categories: Coal
Tags: Philip Kiffer —
May 1st, 2008 · Comments Off on What’s the Controversy? ·
Polyurethane | History of Polyurethane | Chemistry of Polyurethane | Polyurethane Affects History | What’s the Controversy?
JMole Image:
Despite Polyurethane’s plethora of uses, it has not completely escaped controversary.
Here’s an examples:
– In 2004, Massachusetts State Representative Michael Connolly introduced a bill to the Massachusetts State Legislature proposing a ban on the use of all polyurethane based materials in public buildings (including the replacement of existing material) in reaction to the Station Nightclub fire in Warwick, RI, which occurred when a pyrotechnics show ignited the nightclub’s flammable sound-proof polyurethane foam.
-The Alliance for the Polyurethanes Industry, fearing an industry wide backlash, met with Rep. Connolly and testified before the Massachusetts State Legislature to attest to the numerous beneficial uses of polyurethane building materials. Together they addressed Connolly’s concerns by eastablishing science-based standards for the proper use of acounstic materials in public places as well as a fire safety advisory commitee for the incorporation of flame-retardent material into state building codes.
-As a result of these measures, the bill was dropped.
Categories: Polyurethane
Tags: Joshua Wright —
May 1st, 2008 · Comments Off on Polyurethane Affects History ·
Polyurethane | History of Polyurethane | Chemistry of Polyurethane | Polyurethane Affects History | What’s the Controversy?
Polyurethane Affects History – Industrial Uses
–The Polyurethane Industry is a a $41 billion enterprise and a key element of the U.S. economy. The Polyurethane Industry employs more than 263,000 Americans, operates in over 850 locations in the U.S., and helps create nearly 5 jobs for each job in the polyurethanes industry.
Industrial Uses:
| Application |
Amount of polyurethane used(millions of pounds) |
Percentage of total |
| Building & Construction |
1,459 |
26.8% |
| Transportation |
1,298 |
23.8% |
| Furniture & Bedding |
1,127 |
20.7% |
| Appliances |
278 |
5.1% |
| Packaging |
251 |
4.6% |
| Textiles, Fibers & Apparel |
181 |
3.3% |
| Machinery & Foundry |
178 |
3.3% |
| Electronics |
75 |
1.4% |
| Footwear |
39 |
0.7% |
| Other uses |
558 |
10.2% |
| Total |
5,444 |
100.0% |
Categories: Polyurethane
Tags: Joshua Wright —
May 1st, 2008 · Comments Off on Chemistry of Polyurethane ·
Polyurethane | History of Polyurethane | Chemistry of Polyurethane | Polyurethane Affects History | What’s the Controversy?
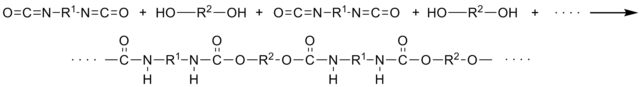
�
or more generally…
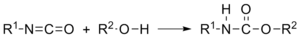
-Polyurethanes are in the class of compounds called reaction polymers, which includes epoxies, polyesters, and phenolics.
– A Urethane Linkage is created by reacting an isocyanate group, -N=C=O, with a hydroxal (alcohol) group, -OH.
-Polyurethanes are produced by the polyaddition reaction of a polyisocyanate with a polyalcohol (polyol) in the presence of a catalyst and other additives.
– In the Polyurethane industry the isocyanate group is referred to as the A-Side, while the blend of polyols and other additives are referred to as the B-Side.
-The chemicals in the B-Side give each specific polyurethane its characteristics; the additions of cross extenders, chain linkers, surfactants, blowing agents, flame retardents, fillers and pigments affect the polyurethane compound created.
Categories: Polyurethane
Tags: Joshua Wright —
Polyurethane | History of Polyurethane | Chemistry of Polyurethane | Polyurethane Affects History | What’s the Controversy?
History of Polyurethane
-First developed by Dr. Otto Bayer in 1937 at the I.G. Farben Laboratories, a subdivision of Bayer Corporation, in Leverkusen, Germany.
-Realized they could create new materials with special characteristics by applying the principle of polyaddition to liquid diisocyanates and existing polyester and polyether diols.
-Part of a larger governement sponsored scientific and technological movement during WWII to create cheap, mass-producable synthetic plastics and rubbers.
-The origin of polyurethane dates back to the beginning of World War II where it is was first developed as a replacement for rubber. The versatility of this new organic polymer and its ability to substitute for scarce materials, spurred numerous applications. During World War II, polyurethane coatings were used for the impregnation of paper and the manufacture of mustard gas resistant garments, high-gloss airplane finishes and chemical and corrosion resistant coatings to protect metal, wood and masonry.
-First commercially available polyurethane was introduced by DuPont Corporation in 1948, material was a rigid foam used in insulation.
-Dow Chemical, BASF, and Mobay Corporation in the following year, 1949, introduced synthetic rubber, polyurethane materials.
-Over the 1960s, through the addition of various additives, chemists were able to develop more flexibile foam polyurethanes, as well as more rigid, hard plastics.
-First all plastic car (made from polyurethanes..!) introduced in 1969 by Bayer AG Corporation in Dusseldorf, Germany.
-Pontiac introduced the first all plastic car in the United States in 1983 using PU materials.
-Increasing use over the 1980s as rising energy costs made it desirable to decrease use of PVC, one of the most common synthetic building materials in the world.
-Development in the 1990s focused on Polyurethane’s potential as a spray sealant. Polyurethane sealants are desriable because of their cheap, easy application, fast drying time, ability to bind to concrete and steel surfaces, and their impermeability.
-Beginning in the early 2000s, industry efforts to become more environmentally friendly created polyurethanes made from vegetable oil polyols, most notably a soy-based polyurethane used by Ford Motor Company in recent automobile interiors (dashboards, side-panels, etc.).
Timeline:
Timeline of Polyurethane Applications
1937
Dr. Otto Bayer discovers the basic polyurethane chemistry. I.G. Farben (Bayer) patents the process
1940
Rigid foam first introduced for aircraft
1941
Adhesive between rubber, metal and glass
1948
First insulation application – a beer barrel
1949
Vulcanized rollable polyurethane rubber
1953
Shoe soles – Synthetic leather
1954
Foam cushions
1958
Introduction of Spandex fiber
1960
Steel sandwich building panels
1966
Integral skin for armrests and shoe soles
1969
Automobile bumpers
1970
Imitation wood
Orthopedics and medical applications
1979
Spray building insulation
1981
Surfboards
1985
Energy absorbing foams for passenger safety
1993
Thin wall medical hoses i.e. catheters
1995
Bicycle tires
2001
Automobile tires
Categories: Polyurethane
Tags: Joshua Wright —
May 1st, 2008 · Comments Off on Polyurethane ·
Polyurethane | History of Polyurethane | Chemistry of Polyurethane | Polyurethane Affects History | What’s the Controversy?
An Introduction to Polyurethane
-Polyurethanes are a class of polymers consisting of organic chains joined by urethane links
-Commonly abbreviated, PU
-Commonly (and misleadingly!) referred to as “urethane”, a mistake since polyurethane’s are different than the actual compound Urethane.
-Developed as a wide-range of materials: low- and high-density foams; soft, flexible solids (like rubber); and firm, rigid plastics.
-Used in everything from shoe soles, golf balls, and watch bands, to automobiles, airplanes, electronics, and even sealants and varnishes.
One of it’s many Uses:

Categories: Polyurethane
Tags: Joshua Wright —
Introduction to Psilocybin – Effects of Psilocybin – History of Psilocybin
Chemistry of Psilocybin

From a clinical standpoint there are few signs that come along with the effects of psilocybin of the body other than irregular and unusual behavior.
· Most physical effects appear to be due to the stimulation of the nervous system while the motor skills decrease in efficiency.
· Increased heart rate appears in less than half of those examined while under the effects of psilocybin.
· Dilated pupils are present in over ninety percent of those examined. Along with this comes enhancement of the deep tendon reflexes found in more than half of those observed.
The hallucinations are very variable depending on the user’s mindset and the dose of the drug ingested The following is an outline of factors that influence the effects of psilocyibin:
· The circumstances under which the mushrooms are used, (e.g. At home, in a group, alone, or as part of a ritual or ceremony)
· The physiological makeup of the individual, including his or her cultural heritage
· Previous experiences with mushrooms and the current expectations
· Dose
· Method of preparation of the fungi (Benjamin, 1995, p. 330)
There are many variables that come into play during a “trip” that determine the overall experience.
Timeline for Psilocybin and its Effects
· 0-30 minutes: Anxiety and tension develop; there may be light-headedness, mild abdominal pain, nausea, weakness, shivering, and muscle aches. The lips may feel numb.
· 30-60 minutes: Onset of visual effects and distortion, development of euphoria and an introspective state; increases in auditory acuity, sweating, tearing, and facial flushing; inability to concentrate; depersonalization; incoordination; tremulous speech; feeling of unreality.
· 1-2 hours: Visual effects intensify, greater distortion of perception; alteration of time sense; continued euphoria, rumination and introspection.
· 2-4 hours: gradual reduction of symptoms, with complete resolution in the majority of individuals. The subject is generally completely normal after 4-8 hours. A few people will have headaches or fatigue, and some experience a more subtle sense of well-being for a number of days.
The duration of the effects of an average dose of psilocybin mushrooms used for “recreational” purposes usually ranges from four to five hours. Headache and hangover are quite uncommon. A feeling of peace and serenity for a number of days after also is not uncommon. In a few subject the flashback has been described, and can continue sporadically in very short spurts, for a number of months. Deaths and dependency directly related to psilocybin rarely occur
Categories: Psilocybin
Tags: Paul Serpe —
Introduction to Psilocybin – Effects of Psilocybin – History of Psilocybin
Chemistry of Psilocybin
Hallucinogenic mushrooms containing psilocybin have probably existed longer than the human race. Throughout history, ancient pictures, carving, and statues have been seen, showing the importance of these fungi to ancient tribes. In Central and South America, use of psilocybin mushrooms was common religious practice until the arrival of Spanish settlers, who were intent on spreading the Catholic faith, strictly prohibited their use. For Indians the fungi are known as sacred mushroom and historically, it is considered entheogen, propelling them on a religious path to the spirit world.
-
Mushroom art and sculptures that depicted motifs under the cap of a mushroom were found in times as early as 1000-500 BC.
-
The purpose of these sculptures and artifacts was not certain, but it is speculated that the stones had religious meaning
-
In sixteenth century Europe alcohol was widely known and used, and even though used symbolically in religious service, served no real purpose in religion.
-
During the Spanish conquest of Central America stories began to come back telling of the use of inebriants in religion.
-
Three substances were mention, one of which was a sacred mushroom called teonanacatl, as well as peyote and the morning glory flower
-
The Codex Vienna Mixtec manuscript that dated back to thirteen century depicted the ritual use of the sacred mushrooms by the Mixtec Gods.
-
The God known as Seven Flowers was the Mixtec God of hallucination, especially in regards to mushrooms, and he was often depicted carrying a pair of mushrooms in his hands
-
Sadly with the conquest of Central America and the integration of Catholicism into the culture these mushrooms were nearly all but lost (Lincoff & Mitchell, 1977).
At the beginning of the twentieth century interest in hallucinogenic plants reappeared.
- The first hallucinogen to be confirmed as such was the peyote cactus.
- At first there was little evidence that morning glory or mushrooms were hallucinogenic, and it was assumed that peyote could be attributed as the reason for all early accounts.
- Just prior to World War II it was found that extracts of the morning glory seed had a depressive effect on the nervous system and ethnobotanists Schultes and Reko discovered these seeds were still in use by local “doctors” in Mexico.
- They traveled to Mexico and sought out these mushrooms and Schultes published a report of his findings in Harvard University Botanical Museum Leaflets in 1939.
This is when and why the use of hallucinogenic fungi was rediscovered
-
After hearing of Schultes and Reko and their finding the hallucinogenic mushrooms in Mexico the ethnomycologists Wasson and Heim went to Central America to investigate the use and effects of these “magic mushrooms”.
-
They published their findings in Life magazine in 1957 their use of the term “magic mushroom” made the term a household name.
-
These mushrooms became a symbol of counterculture in the late 1960’s and 19670’s and became widely used in the United States and Britain. There are no other types of mushrooms that are capable of reflecting the society, culture, and its values the way these mushrooms did at that time. These psilocybin mushrooms lead to the discovery of LSD, a man made chemical hallucinogenic.
-
Today there is still fairly high usage of the drug, and a recent Danish study reported that about 7% of all students have had an experience with psilocybin mushrooms.
-
In another study of drug abusing teenagers, 26% reported using “magic” mushrooms
-
Psilocybin mushrooms are now a Schedule I illicit substance in America, under the Controlled Substances Act, making it completely illegal 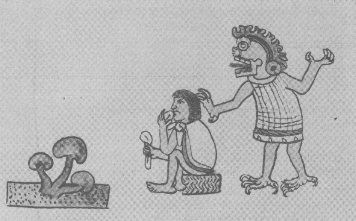
Categories: Psilocybin
Tags: Paul Serpe —
Introduction to Psilocybin – Effects of Psilocybin – History of Psilocybin
Chemistry of Psilocybin
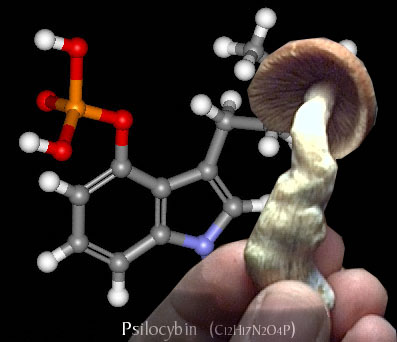
Not long after the initial discovery of magic mushrooms and their effects, that these hallucinogenic fungi before the chemistry involved with these experiences and the drug was examined under a microscope.
· The main type, Psilocybe genus, of mushrooms was found to contain the active ingredients of psilocybin and psilocin.
· After examination a variety of four and five hydroxylated N-methyeltryptamines were isolated from the mushrooms.
· The two compounds, psilocybin and psilocin, are equally psychoactive. Colorless psilocybin can be rapidly oxidized to reveal a blue colored product, which is one of its most common field identification features.
Molecules
· One molecule of psilocybin (O-phosphoryl-4-hydroxy-N) can be broken down into one molecule of psilocin (4-hydroxy-N, N-dimethyl-tryptamine).
· Psilocybin is quickly dephosphorylated to its phosphate ester, psilocin, by alkaline phosphatase, an enzyme present both in the brush border of the intestines and in the liver.
· This removes the phosphate group from psilocybin then leaving, psilocin to be passed along through the bloodstream.
· This breakdown occurs when the mushroom is orally ingested In the Body
· Within thirty minutes the toxins from the drug will have equilibrated, although it accumulates in the liver and adrenal glands.
· This accumulation in the liver and adrenal glands continues for up forty right hours, which could be a very good explanation for the prolonged euphoric sensation that is experienced by many after an ingestion of mushrooms.
· It is interesting that monoamine oxidase, the enzyme normally responsible for the metabolism and degradation of other biogenic amines, such as serotonin, plays almost no role in the elimination of psilocin.
· Usual tryptamine derivatives absorbed from the digestive tract, such as serotonin, do not normally cross the blood-brain barrier. · Psilocin is believed to cross into the brain, most likely due to its high lipid solubility and monoamine oxidase’s failure to affect it
· Structural similarities to the biogenic amines responsible for neurotransmission, such as dopamine and serotonin, suggest that the effects of psilocin are passed through the same pathways, perhaps by “antagonizing” serotonin
· It has also been considered that psilocybin and psilocin, “like other hallucinogenic drugs analogous to serotonin, have serotonin-like and anti-serotonin activities. Possibly their effects on the mind could be related to these actions.”
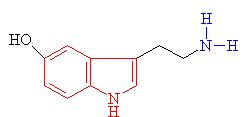
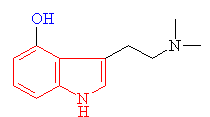
Serotonin
The indole ring is shown in red
and the amine group in blue |
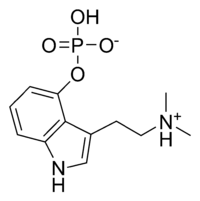
Psilocin
The hydrolysed form of psilocybin
with the change shown in blue |
· As you can see above the two molecules both contain indole rings, which are a 6- membered benzene ring fused to a 5-membered ring containing nitrogen.
· The only difference is the addition of methyl groups. This makes the molecule more lipophilic, soluble in fats, in turn, better able to penetrate the fatty membranes that protect nerves and nerve endings.
· This allows the molecules to easily penetrate the central nervous system, making them more potent.
· Psilocybin has this indole ring, with a phosphoric acid group attached to it. In the body the phosphoric acid group is oxidized to the hydroxyl compound, psilocin, as previously discussed
Categories: Psilocybin
Tags: Paul Serpe —