Entries from April 2008
April 23rd, 2008 · Comments Off on Conclusions
Introduction | What is Caffeine: Molecule Structure | Stimulating Science: The Properties of Caffeine | Discovery: The Magical Bean | Coffee Creates a Social Lifestyle in Europe |Colonization and Coffee|Coffee’s Impact on the Nation of Brazil|Coffee Industry Today| Conclusions
The caffeine molecule has had a profound effect on the history of our world. The drug that has beneficial and stimulating properties has been a staple in society over the centuries. Caffeine, in the form of coffee, has continuously impacted social, economical, and political thoughts and actions. From the first uses of coffee in the Arabic world, to the coffeehouses of Europe, to colonization, to the development of countries such as Brazil, to today where the coffee industry is one of the most large and powerful markets in the world – caffeine has never ceased to influence history. Caffeine, and coffee, will continue to effect and shape the future of the world in the centuries to come.
[Read more →]
Categories: Caffeine
April 23rd, 2008 · Comments Off on About the Site
“Most of us never give a thought to the history or nature of spices or rubber or nicotine or penicillin or a score of other things – chemicals – that have changed the world…” -Oliver Sacks
 “The idea that momentous events may depend on something as small as a molecule – a group of two or more atoms held together in a definite arrangement – offers a novel approach to understanding the growth of human civilization. A change as small as the position of a bond – the link between atoms in a molecule – can lead to enormous differences in properties of a substance and in turn can influence the course of history.”
“The idea that momentous events may depend on something as small as a molecule – a group of two or more atoms held together in a definite arrangement – offers a novel approach to understanding the growth of human civilization. A change as small as the position of a bond – the link between atoms in a molecule – can lead to enormous differences in properties of a substance and in turn can influence the course of history.”
-from “Napoleon’s Buttons”
Professor Samet’s course, titled Chemistry 111: Role of Chemistry in History, is designed for non-science majors. The focal point of the course is a wonderful book titled “Napoleon’s Buttons: How 17 Molecules Changed History (Penny Le Couteur and Jay Burreson; Penguin Putnam Inc., New York, 2003).”
The goal of the course is to compile our own book, with each student contributing a chapter that focuses on a specific molecule. This website, created by the Spring 2008 class, serves as our molecule museum, and we welcome you to visit!
[Read more →]
Categories: Uncategorized
April 23rd, 2008 · Comments Off on Side Effects
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
As with any drug, ibuprofen has some side effects. The list can be long, but some of the more common side effects are listed below.
- chest pain, weakness, shortness of breath, slurred speech, problems with vision or balance
- black or bloody stools
- swelling, rapid weight gain
- less urinating than usual
- nausea, fever, stomach pain
- sore throat, bruising, headache, chills
There have been an increasing number of ibuprofen overdose cases since the drug has become sold over-the-counter. Nevertheless, life-threatening cases of overdose have been low.
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on How is Changed History
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
There are numerous ways in which ibuprofen changed history, but some of the more notable are:
- Because the drug is nonsteroidal, and therefore does not upset the hormonal balance in the body, it can be used extensively. This allowed it to substitute other forms of drugs that were used in the past, which might have been harmful to the body.
- In certain studies, ibuprofen has shown better results than a placebo in the prevention of Alzheimer’s disease when given in small amounts over a long stretch of time.
- Ibuprofen has shown to lower the risk or even prevent Parkinson’s disease, while other drugs such as Aspirin did not have this effect.
- A research study has showed that ibuprofen works best compared to common pain killers such as generic acetaminophen and codeine for kids with broken bones, bruises, and sprains.
- Research shows that use of ibuprofen might lessen the chance of cancer and benign prostatic hyperplasia. However, it is not recommended to take ibuprofen for such purposes, but this phenomenon has rather appeared as a side effect in patients who were prescribed the medicine for other purposes.
- Most commonly,
 ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on Terms
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
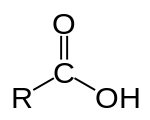 Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.
Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.

Phenyl group = functional group in which 6 carbon atoms are arranged in a cyclic ring structure.
Enantiomer = isomeric molecules with chiral properties with a mirror image relationship.
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on How it Works
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen functions in such a way that it stops the action of the enzyme cyclooxygenase. This enzyme brings about the transformation of fatty acids to prostaglandins. Therefore, ibuprofen stops the synthesis of prostaglandin, which causes its analgesic (pain-relieving) and anti-inflammatory action. More simply said, ibuprofen works by reducing the specific hormones that cause the undesired inflammation and pain in the body.
[Read more →]
Categories: Ibuprofen
April 23rd, 2008 · Comments Off on Chemical Structure
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen’s chemical structure is C13H18O2.
The molecule is a white crystalline powder and has a melting point between 74°C to 77°C. It is not very soluble in water but is soluble in ethanol and acetone.
The molecule is composed of a carboxylic group in the right hand corner, of a phenyl group in the middle, and of an isobutyl group to the left of the image.
Ibuprofen is made up of two separate molecules — R-ibuprofen and S-ibuprofen. These have the same structure but vary in their arrangement. In fact, they are isomers called enantiomers. The difference lies in how the atoms are connected in the second carbon from the right.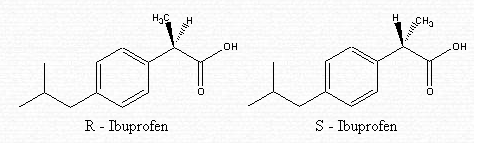
In the R-isomer, the methyl (CH3) group is in the front . In the S-isomer, the methyl (CH3) group is in the back.
[Read more →]
Categories: Ibuprofen
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References

The discovery of ibuprofen was not accidental unlike many other molecules. Its initial purpose was to treat arthritis.
It was discovered in the 1950s by Boots Group, a pharmacy chain in the United Kingdom. It was discovered by Stewart Adams and his colleague John Nicholson. Interestingly enough, Adams first tested the drug on a hangover. The drug was made available to the public almost 20 years later in 1969. During the 1970s ibuprofen would become more popular all over the world. Finally, between 1978 and 1983 the drug started to be sold over-the-counter worldwide, and in 1984 the United States recognized it as an over-the-counter drug as well.
[Read more →]
Categories: Ibuprofen
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen is a drug that contains non-steroidal anti-inflammatory properties as well as analgesic (pain relieving) and fever reducing properties. It is most commonly sold under the trademarks Advil and Motrin. Most commonly, ibuprofen is seen as an over-the-counter drug, but it may also be sold under a prescription if larger doses are necessary.
It is used to temporarily relive minor pains such as:
- Headaches

- Muscular aches
- Minor pain or arthritis
- Toothache
- Backache
- Colds
- Menstrual cramps
- Temporarily reduces fever
The chemical name of ibuprofen is 2-(4-isobutylphenyl) propanoic acid.
Over-the-counter doses of ibuprofen are considered to be low, and these usually range between 200 mg to 400 mg. The duration of such a dose lasts on average between 4 to 8 hours.
[Read more →]
Categories: Ibuprofen
Introduction to Coal | Coal as a Fuel|Coal Affects History| Coal Mining and Effects| Environmental Effects| History Affects Coal|References|
Coal mining is the primary means by which coal is extracted from the ground for use as a fuel or other means. Some coal is found on or close to the surface which is likely how pre-historic man was able to obtain coal for use in campfires. However, by 10,000 B.C. in China, and prior to Roman times in Britannia digging pits and primitive mining techniques became common means of coal production. Extensive mining projects developed in Britian and other countries during the industrial revolution and with them a number of social and environmental problems as miners and the regions surrounding the mines suffered the negative side effects of coal mining.
Mining Techniques used in the Early 19th Century
-
Deep Shaft mining: The most common form of mining during the industrial revolution. The method was relatively primitive and involved non-mechanized workers sheering coal of the mine walls with pick axes and other tools. Deep shaft mining was extremely hazardous and resulted in the death of many workers via accident or illness.
-
Blast mining: Another older technique where dynamite or other explosives blow up target areas of the mine. The coal is then collected and transported out of the mine. Obviously, it’s an extremely hazardous technique.
Dangers and Risks
During the industrial revolution coal mining was an extremely hazardous occupation. Miners were subject to many on-site hazards and health risks. For centuries many miners developed fatal or potentially fatal lung diseases from inhaling soot and toxic gasses in the mines. These diseases included black lung, tuberculosis, and lung cancer.

Additionally, the potential for accidents was usually high. Sloppiness and carelessness sometimes led to collapse or partial collapse of mines (known as mine wall failures). Abuse and misuse of cars and other equipments were other causes of accidental deaths. Perhaps the greatest risk to miners was the buildup of hazardous gasses (called damps) which are the product of various reactions with the coal.
-
Black Damp: CO2 and N → causes suffocation
-
After Damp: Similar to Black Damp → causes explosions
-
Fire Damp: Methane → flammable
-
Stink Damp: Mostly Sulfur → causes explosions
-
White Damp: → Air with a high concentration of CO → highly toxic
Such gasses are responsible for some of the worse mining disasters in history including the Senghenydd Colliery Disaster which occurred in South Wales in 1913. An explosion in the mine believed to be caused by fire damp killed 439 miners, some boys.

Coal Dust Expolosion
[Read more →]
Categories: Coal


 ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking. Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.
Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.





