April 18th, 2008 · Comments Off on Uses 2 ·
Introduction – Discovery – How is it Made?
Uses 1 – Uses 2 – Uses 3
Hydrogen Peroxide and History – Hydrogen Peroxide and the Future

Rocket Fuel
The Messerschmitt 163 rocket aircraft (“Komet”) wasn’t a conventional jet aircraft, it was the only rocket aircraft to ever fly in operational service in WW2. It was fitted with a rocket motor that made it faster than any other fighter in the air, but the fuel made it very unsafe. The fuels were “T Stoff” (80% H2O2, 20% water) and “C Stoff”. It is said that any organic matter (including humans) could spontaneously combust in contact with the T Stoff. The fuel was loaded separately, with the aircraft and crew washed down carefully after the first fuelling, and again after the second one. A catalyst (Ca(MnO4)2 + K2CrO4) was mixed with some peroxide, generating a mixture of steam and oxygen that drove the pumps feeding the two fuels to the combustion chamber. The explosive reaction produced a mixture of hot gases, steam and nitrogen:
2 H2O2 (l) + N2H4 (l) –> 4 H2O (g) + N2 (g)
Unfortunately, if a Me163 crash landed and somehow did not explode (they usually did), fuel lines could fracture, and the pilot would be dissolved alive by the T Stoff.
Categories: Hydrogen Peroxide
Tags: Dana Bunting —
April 18th, 2008 · 1 Comment ·
Introduction – Discovery – How is it Made?
Uses 1 – Uses 2 – Uses 3
Hydrogen Peroxide and History – Hydrogen Peroxide and the Future
Around 2 million tons of hydrogen peroxide are made each year. About half of that is used to bleach wood pulp or paper. It is seen as an environmentally friendly alternative to chlorine-based bleaches. In domestic uses, it is used as a mixture with NaOH to bleach wooden surfaces. It is also used to bleach textiles.
Medically, hydrogen peroxide is used to disinfect skin, as it can kill bacteria by using its oxidizing power. When it comes into contact with a cut, it fizzes as oxygen is released, probably due to the action of catalase in the blood. Dilute H2O2 solution (around 3%) is used as a mouthwash, and also for cleaning and disinfecting contact lenses.
It also bleaches teeth and is a key ingredient in making glow sticks!

Controversially, it has been suggested that use of hydrogen peroxide solution – for example in very low concentrations intravenously – can be used as a therapy for cancer (“oxygen therapy”). The American Cancer Society says that there is nothing to support this, and that it may well be harmful.
Fun Fact: Hydrogen peroxide, if spilled on clothing (or other flammable materials), will preferentially evaporate water until the concentration reaches sufficient strength, then clothing will spontaneously ignite.
Categories: Hydrogen Peroxide
Tags: Dana Bunting —
April 18th, 2008 · Comments Off on Discovery (and Properties) ·
Introduction – Discovery – How is it Made?
Uses 1 – Uses 2 – Uses 3
Hydrogen Peroxide and History – Hydrogen Peroxide and the Future
Hydrogen Peroxide was first dicovered by Louis- Jacques Thenard in 1818 by burning barium salts to create barium peroxides. When the Barium peroxides were put into water to dissolve, Thenard found that hydrogen peroxide was produced.
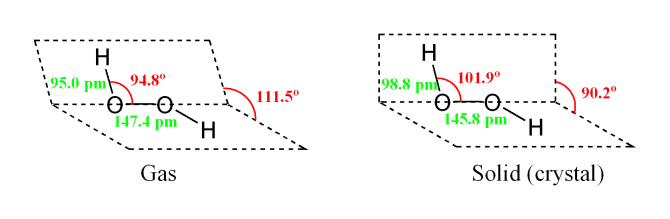
Hydrogen Peroxide is a is a strong oxidizing agent and a weak acid in water solution. The formula is similar to that of water, with an extra atom of oxygen attached, H2O2. It is completely soluble in water. Pure anhydrous hydrogen peroxide is a colorless to pale blue syrupy liquid which decomposes violently into water and oxygen if heated above 80 C. it also decomposes in light and in the presence of metal ions or oxidizable organic materials. One volume of hydrogen peroxide releases ten volumes of oxygen when it decomposes. The most valuable property of hydrogen peroxide is that it breaks down into water and oxygen and therefore does not form any persistent, toxic residual compounds.
Categories: Hydrogen Peroxide
Tags: Dana Bunting —
April 18th, 2008 · Comments Off on Introduction ·
Categories: Hydrogen Peroxide
Tags: Dana Bunting —
April 17th, 2008 · Comments Off on Pregnancy ·
Introduction to AZT | The Chemistry of AZT| Side Effects| Pregnancy| An Anything But American Virus| Opposing Views| How AZT Changed History|
- In the U.S., about 25 percent of pregnant HIV-infected women not receiving AZT therapy passed on the infection to their babies. AZT reduced that 25 percent to 8 percent

- The treatment cost per pregnant woman is still $1,000
Categories: AZT
Tags: Tynesha Wright —
April 17th, 2008 · Comments Off on How AZT Changed History ·
Introduction to AZT | The Chemistry of AZT| Side Effects| Pregnancy| An Anything But American Virus| Opposing Views| How AZT Changed History|
- Nearly doubled the life expectancy for persons with AIDS
- Can reduce maternal transmission of AIDS during pregnancy by 2/3
- Too toxic for cancer in 1964 but worked 20 years later with antiviral therapy
- March 1987 “The world of clinical research was turned upside down” (National
Academy of Sciences) when the FDA approved AZT after phase one instead of the usual phase 3
- $10,000 annually-one of the most expensive drugs in history

Categories: AZT
Tags: Tynesha Wright —
April 17th, 2008 · Comments Off on Conclusion ·
Intro | Chemistry | Sources | Affects History | History affects | Poor Countries | Undesired Effects | Substitutes | Conclusion
Quinine, in sum, is one the molecules that have had a tremendous impact on the history of mankind. Although its medicinal properties were known well before, it has been made popular thanks to the campaign of the Jesuits in the 17th century. The understanding of its chemistry as well as periods of high demands have led to attempts towards its synthetic make up which has only been achieved in 2001. Because of its many side effects and the scarcity of its source, cheaper and better alternatives have been sought after. These substitutes, however, did not completely remove quinine from the list of the leading drugs for the treatment and prevention of the world number one killer disease. After the recent successful synthesis of this potent molecule, there is reason to believe that one day soon, the world may free itself from the malaria disease.
Bibliography:
- Ball, Phillip. “History of science: Quinine steps back in time”. Nature: 2008. www.nature.com.
- Barton, Patricia & Mills, James H. Drugs and empires: Essays in Modern Imperialism and Intoxication. Palgrave McMillan. New York: 2007.
- Burreson, Jay & Le Couteur, Penny. Napoleon’s button: 17 molecules that changed history. Jeremy P. Tarcher/Penguin. New York: 2004.
- Considine, Glenn D. Van Nostrand’s encyclopedia of chemistry. Wiley-Interscience. Hoboken, NJ: 2005.
- Cotton, Simon. “The Mighty Quinine”. http://www.chm.bris.ac.uk. http://www.chm.bris.ac.uk/motm/quinine/quininev.htm
- Dagani, Ron. Quinine. Chemical & Engineering News. Washington, DC: 2005. http://envoy.dickinson.edu:2048/login?url=http://proquest.umi.com/pqdweb?did=857229151&sid=3&Fmt=2&clientId=4534&RQT=309&VName=PQD
- Duran-Reynals. The fever bark tree. Double Day and Company. Garden City: 1946.
- Frankel, S. Herbert. The economic impact on underdeveloped countries: Essay on international investment and social change. Harvard University Press. Cambridge: 1955.
- Isaacman, Allen & Roberts, Richard. Cotton, Colonialism, and Social History in Sub-Saharan Africa. Heinemann. Portsmouth, NH: 1995.
- Keen, Mary. The dark side of the vegetable world. The Spectator. London: 1999. http://envoy.dickinson.edu:2048/login?url=http://proquest.umi.com/pqdwep?did=42416027&sid=1&Fmt=3&clientId=4534&RQT=309&VName=PPQ
- Magill, Alan & Panosian Claire. Making Antimalarial Agents Available in the United States. The New England Journal of Medicine. Boston: 2005. http://envoy.dickinson.edu:2048/login?url=http://proquest.umi.com/pqdweb?did=875776471&sid=8&Fmt=3&clientId=4534&RQT=309&VName=PQD
- Mahar, Dulcy. Homes & Garden of the Northwest. The Orgonian. Portaland, Or: 2003. http://envoy.dickinson.edu:2048/login?url=http://proquest.umi.com/pqdweb?did=489774871&sid=1&Fmt=3&clientId=4534&RQT=309&VName=PQD
- Rinsky, Rober A. Public Health Reports: Historical Collection 1878-2005. Association of Schools of Public Health. Washington DC: 2005.
- Werner, Louis. Quinine’s: Feverish tales and trails. Americas. Washington, DC: 2003. http://envoy.dickinson.edu:2048/login?url=http://proquest.umi.com/pqdweb?did=419274271&sid=2&Fmt=4&clientId=4534&RQT=309&VName=PQD
- Williams, Stephen. The Fever Trail. African Business. London: 2002. http://envoy.dickinson.edu:2048/login?url=http://proquest.umi.com/pqdweb?did=105869691&sid=1&Fmt=4&clientId=4534&RQT=309&VName=PQD
Categories: Quinine
Tags: Moustapha Minte —
April 17th, 2008 · Comments Off on Substitutes of Quinine ·
Intro | Chemistry | Sources | Affects History | History affects | Poor Countries | Undesired Effects | Substitutes | Conclusion
Over the past few decades, chloroquine-resistant strains of the malaria parasite have spread very rapidly. This has reduced the effectiveness of chloroquine and other quinine related drugs.
Drugs like fansidar, and mefloquine are often used as substitutes.
These drugs, however, are highly toxic with, sometimes, allarming side effects.
Therefore, quinine still remains one of the leading drugs for the treatment and prevention of malaria.
Categories: Quinine
Tags: Moustapha Minte —
April 17th, 2008 · Comments Off on Undesired Effects of Quinine ·
Intro | Chemistry | Sources | Affects History | History affects | Poor Countries | Undesired Effects | Substitutes | Conclusion
Quinine may be harmful if wrongly used and/or depending on the tolerability of the patient.
-
Certain patients may vomit after ingesting malaria tablets
-
If accidentally injected in nerves, it can paralyze
-
It is extremely toxic in overdose
-
In the U.S. it is classified as a Category X teratogen by the Food and Drugs Adminsitration: It can cause birth defects if taken by pregnant woman
-
It can be itchy
Categories: Quinine
Tags: Moustapha Minte —
April 17th, 2008 · Comments Off on Quinine in Third World Countries ·
Intro | Chemistry | Sources | Affects History | History affects | Poor Countries | Undesired Effects | Substitutes | Conclusion

Breeding place for mosquitoes
-
Malaria is number one killer in many developping countires
-
Every year hundreds of millions suffer from malaria and 2 to 3 million of them die, mainly in Africa.
Adavantages of Quinine:
-
Quinine still being used in many developping countries as treatment against malaria
-
It is accessible and affordable, but not always reliable.
-
In many countries they have a lot of fake pills.
Disadvantages:
-
The quinine molecule has been instrumental in the exploitation of many developping countries.
-
The bark of the cinchona tree has brought barely any economic benefit to the indigenous people of the Andes.
“Outsiders benefited from the the quinine molecule, exploiting a unique resource of a less developped country for their own advsantage”.
Napoleon’s Buttons: p.348-349.
- Without quinine, most of today’s developping countries would not have been colonized and exploited for so long.
- Finally, despite the existence of quinine and other drugs, malaria still is rife in poor countries.
- There are conditions which facilitate the reproduction of the mosquitoes.
- With such favorable conditions for its development, the parasite develops resitant forms to antimalarial drugs.
Categories: Quinine
Tags: Moustapha Minte —






