April 29th, 2008 · Comments Off on A Fertilizer ·
Introduction | Chemical and Physical Properties | Synthesis of Ammonia: The Haber-Bosch Process | A Fertilizer | Ammonia Affects History | Other Uses
- The main use of ammonia is in fertilizers. Fertilizers are used as a substance added to soil in order to improve the growth and yield of plants. All plants rely on nutrients in the soil in order to survive, most importantly nitrogen. However, as the plants grow they use up those nutrients in the soil. Thus, something is needed in order to replenish those vital nutrients. Fertilizers replace the chemical components that are taken from the soil by growing plants. The replenishing of nutrients in the soil by fertilizers make the plans grow more efficiently.
- Modern-day research into fertilizers began in the early 1600s. Scientists began to discover the chemical needs of plants and thus began to improve fertilizers. Organic chemists Justus con Liebig discovered that plants needed mineral elements such as nitrogen and phosphorous in order to grow. Subsequently, the first patent was issued to John Lawes for his method of producing a form of phosphate that was effective as a fertilizer. Lawes’ patent is often said to have originated the fertilizer industry. The fertilizer industry then experienced its most significant growth after World War I when ammonia facilities that were producing explosives for the war were converted to the production of fertilizers.
- It was this post-war era that saw the growth of fully integrated factories deigned to produce fertilizers. It was quickly realized that ammonia would be a good source for these fertilizers because it could be synthesized from inexpensive raw materials.
Categories: Ammonia
Tags: Steven Meyers —
April 29th, 2008 · Comments Off on Chemical and Physical Properties ·
Introduction | Chemical and Physical Properties | Synthesis of Ammonia: The Haber-Bosch Process | A Fertilizer | Ammonia Affects History | Other Uses
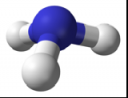
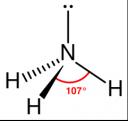
- Ammonia is a colorless gas with a strong, pungent odor
- It is a chemical consisting of one nitrogen atom and three hydrogen atoms (NH3)
- It has a trigonal pyramidal shape with the nitrogen atom at its center
- The nitrogen atom has a lone electron pair
Ball-and-Stick Model of Ammonia:
Molecular Structure of Ammonia:
Categories: Ammonia
Tags: Steven Meyers —
April 29th, 2008 · 1 Comment ·
Introduction | Chemical and Physical Properties | Synthesis of Ammonia: The Haber-Bosch Process | A Fertilizer | Ammonia Affects History | Other Uses
Many would be surprised to hear that one of the most significant and important contributions to the well being of humankind over the past two hundred years has been the industrial synthesis of ammonia. Little do people know that their very existence on this earth today may possibly be a result of ammonia.
Since nitrogen is the crucial element for all living organisms and ammonia is a great source of nitrogen, the synthesis of ammonia was greatly sought after. When its synthesis was finally discovered in the early 1900s, it gave birth to the fertilizer industry.
Fertilizers greatly increased crop yields around the world resulting in massive population increases during the 20th century. In the 1990s, synthetic fertilizers were responsible for producing 40% of the world’s food. That means that without ammonia more than 2.2 billion people would not be on this planet today!
Categories: Ammonia
Tags: Steven Meyers —
April 29th, 2008 · Comments Off on U.S. and the West ·
Introduction | History | How It Works
Uses | Typhoid Fever | Side Effects
Synthetic Production | U.S. and the West
References
The U.S. and the Western world have stopped using chloramphenical in its ingestible form. Only topical ointments are recommended, as for conjuctivitis.
According to Tatli et al. (2003), “approximately 16 million cases of typhoid fever occur annually in the developing world” (p. 350). The U.S. and the Western world is lucky to have the choice of whether or not to use chloramphenicol to fight a widespread epidemic. Many developing countries are afflicted with “civil war,” to suffering economies, and the deterioration of the “country’s infrastructure” (Mermin et al., 1999, p. 1416).
Of those who become victims of typhoid fever, “5%…become asymptomatic chronic carriers” (Mermin et al., 1999, p. 1416).Typhoid fever has influenced history in its shift of power in Ancient Greece and in the persecution of Mary Mallon, a woman whose nickname “Typhoid Mary” is still recognized today. History, however, has also changed medicine and the treatment of disease around the world. The ability to synthetically produce chloramphenicol has allowed underdeveloped nations to have access to an antibiotic that successfully treats typhoid fever. Its controversial side effects are changing the course of history. Some underdeveloped nations refuse to use chloramphenicol because it can cause aplastic anemia and childhood leukemia. This refusal is limiting the ability of developing nations to treat epidemics that exist and may spread due to unchanging sanitation issues. Chloramphenicol has provided the world with the hope to provide affordable healthcare to all nations of the world. The emergence of this antibiotic has made this hope a realization, and has relieved many victims of typhoid fever around the world, no matter economic circumstance.
Categories: Chloramphenicol
Tags: Sarah Williams —
April 29th, 2008 · 1 Comment ·
Introduction | History | How It Works
Uses | Typhoid Fever | Side Effects
Synthetic Production | U.S. and the West
References
Chloramphenicol was the first antibiotic to be produced synthetically in large quantities, making it cheaper than other antibiotics and widely available. Its affordability made it a lucrative asset for many third world countries affected by typhoid.
In chemistry, the synthesis of a molecule is the formation of a complex substance from simpler compounds. Due to its simple structure, chloramphenicol is simple to synthesize.
In his trials in synthesizing chloramphenicol, Gottlieb used a variety of compounds and amino acids, including:
- p-nitrophenylserinol
- dichloroacetic acid
- leucine
- isoleucine
- methionine
- tryptophan
- glutamic acid
- norvaline
- threonine
- phenylalanine
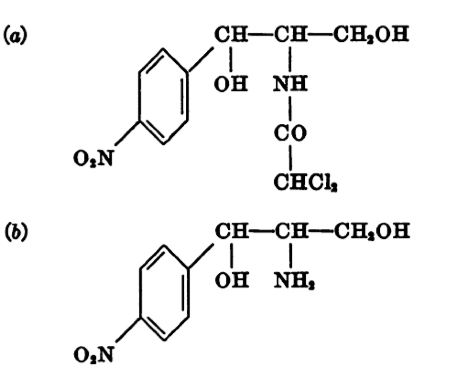
Upon further experimentation, Gottlieb discovered that p-nitrophenylserinol was the best in stimulating antibiotic production.
In this image, the structure of chloramphenicol (a) is compared to the structure of p-nitrophenylserinol (b)
This video clip is a news piece discussing the reasons why Nigerian health officials have decided against the use of chloramphenicol to treat typhoid fever.
Categories: Chloramphenicol
Tags: Sarah Williams —
April 29th, 2008 · Comments Off on Side Effects ·
Introduction | History | How It Works
Uses | Typhoid Fever | Side Effects
Synthetic Production | U.S. and the West
References
- Aplastic Anemia – condition where bone marrow does not produce enough new cells to replace blood cells. It is the most serious side effect of Chloramphenicol. It is rare and fatal because there is no treatment and no conditions of the patient to predict who will be affected. Occurs after the Chloramphenicol treatments have stopped.The risk of developing aplastic anemia following chloramphenicol therapy is “ 1/10,000” (Tatli et al., 2003, p. 350).
- Leukemia. The use of chloramphenicol can induce childhood leukemia is younger patients (Shu et al., 1987, p. 935). The “relapse rate” of typhoid fever is also considered a side effect. The “relapse rate” of typhoid fever after successfully completing chloramphenicol therapy is “approximately 10%,” making the drug less desirable even to underdeveloped countries facing typhoid epidemics (Tatli et al., 2003, p. 350).
Categories: Chloramphenicol
Tags: Sarah Williams —
April 29th, 2008 · Comments Off on Typhoid Fever ·
Introduction | History | How It Works
Uses | Typhoid Fever | Side Effects
Synthetic Production | U.S. and the West
References
Chloramphenicol’s first and foremost use globally was originally to combat typhoid fever.
Ancient Greece
Chloramphenicol was perceived as a miracle cure for the illness because of its rapid success in treating the infection. It was introduced for clinical use in the United States in 1949 after Gottlieb discovered it in a strain of Streptomyces. After this initial introduction, Chloramphenicol spread throughout the West and eventually to the eastern world.
While Chloramphenicol first appeared in the mid-20th century, typhoid fever has existed since around 430 B.C. Caused by the bacterium Salmonella enterica servoar Tyhpi, typhoid occurred in Athens as a mysterious plague. The plague killed nearly one third of the population, including the Athenian leader, Pericles. At the time of the outbreak, the people of Attica were living in tents and Long Walls, promoting poor hygiene and poor public sanitation. Typhoid is mainly transmitted by fecal contaminated drinking water (not washing hands after going to the bathroom).
Typhoid Mary

In 1907, Mary Mallon, an Irish emigrant, was identified as the first asymptomatic carrier of typhoid fever. She had come to the U.S. in 1884 and worked as a cook in New York City from 1900 to 1907. Within two weeks of working in her first household, the residents came down with typhoid fever. The next family she worked for in Manhattan also fell ill, and one member died. In the third house she work for, seven of eight residents became sick with typhoid. The next house she was a cook for in Long Island experienced six cases of hospitalized typhoid in family of eleven. Mallon also infected three households after this.
Mallon was taken into custody by the New York City Health Department and she was quarantined for three years. She was released from quarantine under the condition that she would never again work with food; Mallon did not feel obliged to heed this condition and assumed the name “Mary Brown.” By 1915, after returning to her job as a cook, now at a New York hospital, Mallon had infected 25 more people, two of whom died from typhoid. Health officials returned Mallon to quaratine. Her second time in quaratine was also her last, she committed to quarantine for life.
Upon her death, due to pneumonia, an autopsy was conducted and typhoid bacteria were found in her gall bladder. Mallon was cremated.
Categories: Chloramphenicol
Tags: Sarah Williams —
April 29th, 2008 · Comments Off on Uses ·
Introduction | History | How It Works
Uses | Typhoid Fever | Side Effects
Synthetic Production | U.S. and the West
References
• Typhoid Fever
• Gram-positive bacteria and most strains of MRSA (bacterium responsible for many human infections)
• Conjunctivitis
• Staphylococcal brain abscesses (staph infections)
• Meningitis (permitted in the West when patient has severe allergy to penicillin or cephalosporin)
• Cholera, when resistant to tetracycline (also developed from Streptomyces bacterium, but used more commonly for acne)
Categories: Chloramphenicol
Tags: Sarah Williams —
April 29th, 2008 · Comments Off on How It Works ·
Introduction | History | How It Works
Uses | Typhoid Fever | Side Effects
Synthetic Production | U.S. and the West
References
Chloramphenicol attacks bacterial cell’s ribosomes (protein creators), disturbing the cell’s ability to metabolize and synthesize new protein, and therefore killing the cell. It also stops the cell from dividing, and the immune system is then able to destroy the bacterial cell.
Chloramphenicol is unique in its structure because it “has groups… that are not usually seen in antibiotics” (“Chloramphenicol,” 2007). These groups are the dichloroacetamide group and the aryl nitro group. The aryl nitro group is of specific importance because it has been implicated as the cause of the side effect aplastic anemia. It is theorized that the aryl nitro group is “reduced to form an intermediate” and that this “intermediate… then interacts with the ferrous ion in hemoglobin and oxidizes it to a ferric state” (“Chloramphenicol,” 2007). In this ferric state, the hemoglobin will be unable to carry oxygen, causing anemia. 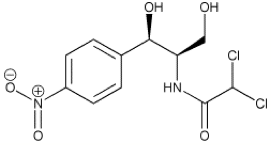
Categories: Chloramphenicol
Tags: Sarah Williams —
April 29th, 2008 · Comments Off on History ·
Introduction | History | How It Works
Uses | Typhoid Fever | Side Effects
Synthetic Production | U.S. and the West
References
In 1947, professor of plant pathology at the University of Illinois David Gottlieb isolated a strain of Streptomyces (a bacteria found in soil), from which Chloramphenicol was developed.
Chloramphenicol was introduced into clinical use in 1948.
Chloramphenicol was seen, upon its discovery, as a near-perfect drug.
• It could be given orally, and at home
• Because of it high pH, it is able to penetrate purulent material (material containing pus), and through cell membranes to parasites and into organs that other antibiotics could not reach
Categories: Chloramphenicol
Tags: Sarah Williams —





