April 23rd, 2008 · 2 Comments ·
Introduction / DDT molecule / Malaria / World War II
Environmental Problems / Insect Resistance / Begin Using Again? / References

DDT was first synthesized by Othmar Zeidler from Strasbourg, Austria in 1874. He was a chemistry student testing various compounds to get material for his doctorate. He recorded the synthesis of DDT in 6 lines in a journal and then forgot about it. It was synthesized again in 1938 by a Swiss chemist named Paul Muller of Basle. He discovered the unique properties that DDT had and realized the importance of such an insecticide. In 1939, Switzerland was having problems importing chemicals due to the beginning of the war and the German invasion of Poland. The Colorado potato beetle struck their potato crop and DDT was first put to the test. It completely destroyed the beetle and save the crop. Then in 1942 the Geigy Company, a Swiss Dye House that owned the patent, offered it to the American Army. After a year long intense investigation and study of the compound by the Bureau of Entomology and Plant Quarantine, DDT was given to the Army to protect against insects found abroad that carried disease.
Categories: DDT
Tags: Kimberly Ward —
April 23rd, 2008 · Comments Off on Conclusions ·
Introduction | What is Caffeine: Molecule Structure | Stimulating Science: The Properties of Caffeine | Discovery: The Magical Bean | Coffee Creates a Social Lifestyle in Europe |Colonization and Coffee|Coffee’s Impact on the Nation of Brazil|Coffee Industry Today| Conclusions
The caffeine molecule has had a profound effect on the history of our world. The drug that has beneficial and stimulating properties has been a staple in society over the centuries. Caffeine, in the form of coffee, has continuously impacted social, economical, and political thoughts and actions. From the first uses of coffee in the Arabic world, to the coffeehouses of Europe, to colonization, to the development of countries such as Brazil, to today where the coffee industry is one of the most large and powerful markets in the world – caffeine has never ceased to influence history. Caffeine, and coffee, will continue to effect and shape the future of the world in the centuries to come.
Categories: Caffeine
Tags: Emily Decatur —
April 23rd, 2008 · Comments Off on About the Site ·
“Most of us never give a thought to the history or nature of spices or rubber or nicotine or penicillin or a score of other things – chemicals – that have changed the world…” -Oliver Sacks
 “The idea that momentous events may depend on something as small as a molecule – a group of two or more atoms held together in a definite arrangement – offers a novel approach to understanding the growth of human civilization. A change as small as the position of a bond – the link between atoms in a molecule – can lead to enormous differences in properties of a substance and in turn can influence the course of history.”
“The idea that momentous events may depend on something as small as a molecule – a group of two or more atoms held together in a definite arrangement – offers a novel approach to understanding the growth of human civilization. A change as small as the position of a bond – the link between atoms in a molecule – can lead to enormous differences in properties of a substance and in turn can influence the course of history.”
-from “Napoleon’s Buttons”
Professor Samet’s course, titled Chemistry 111: Role of Chemistry in History, is designed for non-science majors. The focal point of the course is a wonderful book titled “Napoleon’s Buttons: How 17 Molecules Changed History (Penny Le Couteur and Jay Burreson; Penguin Putnam Inc., New York, 2003).”
The goal of the course is to compile our own book, with each student contributing a chapter that focuses on a specific molecule. This website, created by the Spring 2008 class, serves as our molecule museum, and we welcome you to visit!
Categories: Uncategorized
Tags: Cindy Samet —
April 23rd, 2008 · Comments Off on Side Effects ·
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
As with any drug, ibuprofen has some side effects. The list can be long, but some of the more common side effects are listed below.
- chest pain, weakness, shortness of breath, slurred speech, problems with vision or balance
- black or bloody stools
- swelling, rapid weight gain
- less urinating than usual
- nausea, fever, stomach pain
- sore throat, bruising, headache, chills
There have been an increasing number of ibuprofen overdose cases since the drug has become sold over-the-counter. Nevertheless, life-threatening cases of overdose have been low.
Categories: Ibuprofen
Tags: Toni Bujas —
April 23rd, 2008 · Comments Off on How is Changed History ·
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
There are numerous ways in which ibuprofen changed history, but some of the more notable are:
- Because the drug is nonsteroidal, and therefore does not upset the hormonal balance in the body, it can be used extensively. This allowed it to substitute other forms of drugs that were used in the past, which might have been harmful to the body.
- In certain studies, ibuprofen has shown better results than a placebo in the prevention of Alzheimer’s disease when given in small amounts over a long stretch of time.
- Ibuprofen has shown to lower the risk or even prevent Parkinson’s disease, while other drugs such as Aspirin did not have this effect.
- A research study has showed that ibuprofen works best compared to common pain killers such as generic acetaminophen and codeine for kids with broken bones, bruises, and sprains.
- Research shows that use of ibuprofen might lessen the chance of cancer and benign prostatic hyperplasia. However, it is not recommended to take ibuprofen for such purposes, but this phenomenon has rather appeared as a side effect in patients who were prescribed the medicine for other purposes.
- Most commonly,
 ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
Categories: Ibuprofen
Tags: Toni Bujas —
April 23rd, 2008 · Comments Off on Terms ·
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
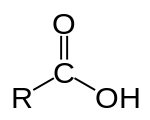 Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.
Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.

Phenyl group = functional group in which 6 carbon atoms are arranged in a cyclic ring structure.
Enantiomer = isomeric molecules with chiral properties with a mirror image relationship.
Categories: Ibuprofen
Tags: Toni Bujas —
April 23rd, 2008 · Comments Off on How it Works ·
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen functions in such a way that it stops the action of the enzyme cyclooxygenase. This enzyme brings about the transformation of fatty acids to prostaglandins. Therefore, ibuprofen stops the synthesis of prostaglandin, which causes its analgesic (pain-relieving) and anti-inflammatory action. More simply said, ibuprofen works by reducing the specific hormones that cause the undesired inflammation and pain in the body.
Categories: Ibuprofen
Tags: Toni Bujas —
April 23rd, 2008 · Comments Off on Chemical Structure ·
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen’s chemical structure is C13H18O2.
The molecule is a white crystalline powder and has a melting point between 74°C to 77°C. It is not very soluble in water but is soluble in ethanol and acetone.
The molecule is composed of a carboxylic group in the right hand corner, of a phenyl group in the middle, and of an isobutyl group to the left of the image.
Ibuprofen is made up of two separate molecules — R-ibuprofen and S-ibuprofen. These have the same structure but vary in their arrangement. In fact, they are isomers called enantiomers. The difference lies in how the atoms are connected in the second carbon from the right.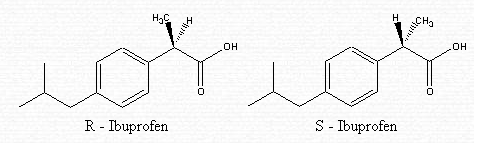
In the R-isomer, the methyl (CH3) group is in the front . In the S-isomer, the methyl (CH3) group is in the back.
Categories: Ibuprofen
Tags: Toni Bujas —
April 22nd, 2008 · 3 Comments ·
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References

The discovery of ibuprofen was not accidental unlike many other molecules. Its initial purpose was to treat arthritis.
It was discovered in the 1950s by Boots Group, a pharmacy chain in the United Kingdom. It was discovered by Stewart Adams and his colleague John Nicholson. Interestingly enough, Adams first tested the drug on a hangover. The drug was made available to the public almost 20 years later in 1969. During the 1970s ibuprofen would become more popular all over the world. Finally, between 1978 and 1983 the drug started to be sold over-the-counter worldwide, and in 1984 the United States recognized it as an over-the-counter drug as well.
Categories: Ibuprofen
Tags: Toni Bujas —
April 22nd, 2008 · 3 Comments ·
Introduction | History | Chemical Structure | How it Works | Side Effects | How it Changed History | Terms | References
Ibuprofen is a drug that contains non-steroidal anti-inflammatory properties as well as analgesic (pain relieving) and fever reducing properties. It is most commonly sold under the trademarks Advil and Motrin. Most commonly, ibuprofen is seen as an over-the-counter drug, but it may also be sold under a prescription if larger doses are necessary.
It is used to temporarily relive minor pains such as:
- Headaches

- Muscular aches
- Minor pain or arthritis
- Toothache
- Backache
- Colds
- Menstrual cramps
- Temporarily reduces fever
The chemical name of ibuprofen is 2-(4-isobutylphenyl) propanoic acid.
Over-the-counter doses of ibuprofen are considered to be low, and these usually range between 200 mg to 400 mg. The duration of such a dose lasts on average between 4 to 8 hours.
Categories: Ibuprofen
Tags: Toni Bujas —



 ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking.
ibuprofen has allowed us to lessen or even altogether eliminate any pains mentioned in the introduction, such as headaches, toothaches, arthritis, temporarily remove fever, and most important of all for college students, it has allowed us to get rid of most hangovers after a night of heavy drinking. Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.
Carboxylic group = a functional group present in amino acids. The group’s structure is composed of one carbon atom attached to an oxygen atom by a double bond and to a hydroxyl group by a single bond.



